Extreme pressure of course and applying a twisting force along with the pressure. All this produces something a little different. All microscopic of course.
This is likely going no where as we have been able to do this forever. Particularly when hte mining industry produces ample small diamonds as a mattert of course. Gem sizes are rare enough, but not so for industrial diamonds.
Interesting variation though..
Scientists produce rare diamonds in minutes at room temperature
By Nick Lavars
November 18, 2020
https://newatlas.com/materials/scientists-rare-diamonds-minutes-room-temperature/?
Study author Xingshuo Huang with the device used to create the lab-grown diamonds
Jamie Kidston, ANU
While traditional diamonds are formed over billions of years deep in the Earth where extreme pressures and temperatures provide just the right conditions to crystalize carbon, scientists are working on more expedient ways of forging the precious stones. An international team of researchers has succeeded in whittling this process down to mere minutes, demonstrating a new technique where they not only form quickly, but do so at room temperature.
Although the idea of creating diamonds in a laboratory in just a few minutes would be an appealing one for jewelers, rappers or those looking to pop a certain question, that’s not quite the aim of this type of research.
Artificial versions of this famously hard material could find use as new cutting tools to slice through ultra-hard materials, new kinds of protective coatings or other industrial devices where hardness is a desirable attribute. And recently we’ve seen some promising techniques developed that can turn fossil fuel molecules into pure diamonds, or make them from carbon nanofibers with the help of superfast lasers.
This latest breakthrough was led by scientists at the Australian National University (ANU) and RMIT University, who used what’s known as a diamond anvil cell, which is a device used by researchers to generate the extreme pressures needed to create ultra-hard materials. The team applied pressure equal to 640 African elephants on the tip of a ballet shoe, doing so in a way that caused an unexpected reaction among the the carbon atoms in the device.
“The twist in the story is how we apply the pressure,” says ANU Professor Jodie Bradby. “As well as very high pressures, we allow the carbon to also experience something called ‘shear’ – which is like a twisting or sliding force. We think this allows the carbon atoms to move into place and form Lonsdaleite and regular diamond.”
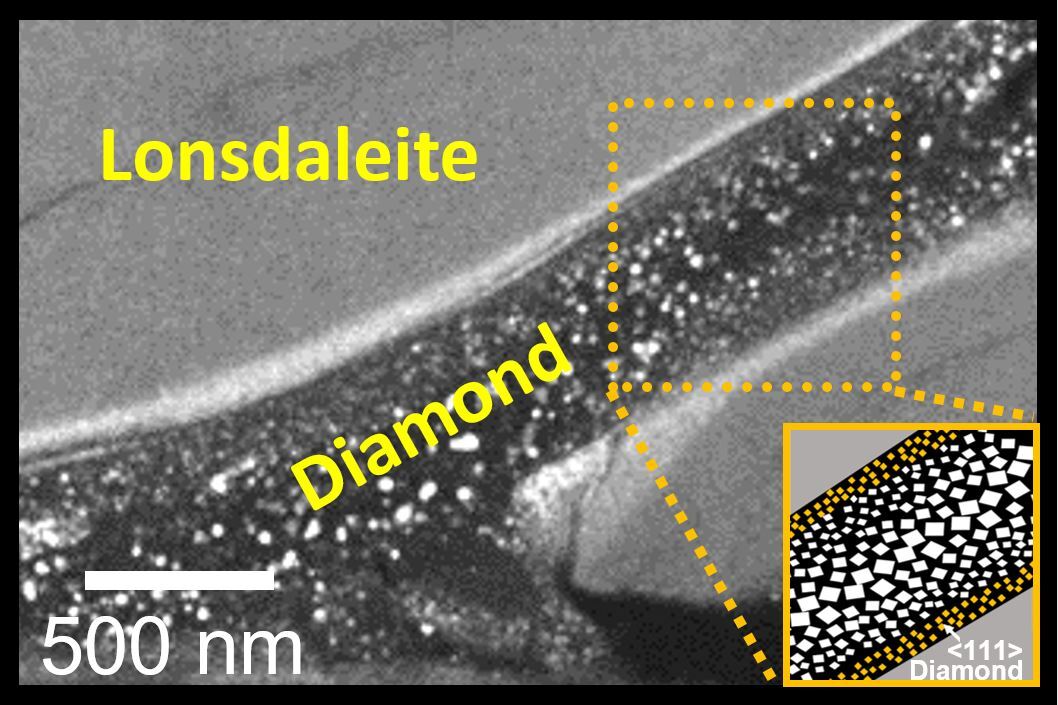
These regular diamonds are the type you might find in an engagement ring, while Lonsdaleite diamonds are rarer and found at meteorite impact sites. Using advanced electron microscopy, the team was able to examine the samples in detail, and found that the materials were formed within bands they liken to “rivers" of diamond.
The team found that their diamonds were formed within bands that they liken to “rivers"
“Our pictures showed that the regular diamonds only form in the middle of these Lonsdaleite veins under this new method developed by our cross-institutional team,” says RMIT’s Professor Dougal McCulloch. “Seeing these little ‘rivers’ of Lonsdaleite and regular diamond for the first time was just amazing and really helps us understand how they might form.”
The team hopes the technique can enable them to produce meaningful quantities of these artificial diamonds, particularly Lonsdaleite, which is predicted to be 58 percent harder than regular diamonds.
“Lonsdaleite has the potential to be used for cutting through ultra-solid materials on mining sites,” Bradby says.
The research was published in the journal Small, while you can hear from the researchers in the video below.
New diamond harder than ring bling
No comments:
Post a Comment