This is a great result and is certainly useful. melanoma is a nasty that often as not recurs.
Savng half of the potential victims is an unexpected gain. Not unlike the AIDs cocktail which ended up providing the victims a full life until old age caught up.
All good and may well do better in general applicationii.
Melanoma recurrence risk cut by 49% by new vaccine/existing drug combo
By Paul McClure
June 03, 2024
https://mail.yahoo.com/d/folders/1/messages/AAU2R7Abz2AzZl7X-wHBOFdwCOw
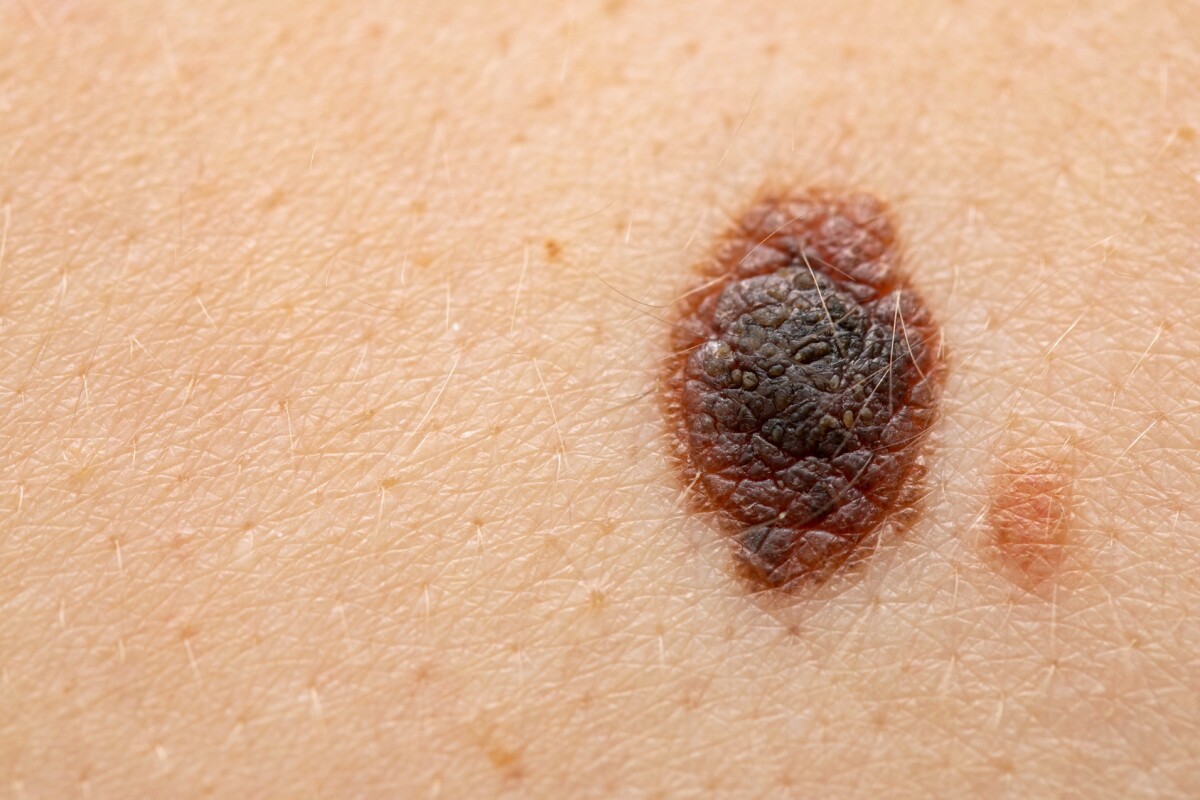
Combining a new melanoma vaccine with an existing drug significantly reduced the risk of the cancer recurring
Results from human trials coupling a new melanoma vaccine with an existing cancer drug showed that, at the three-year mark, the combination treatment reduced the risk of melanoma recurring by 49% and the risk of it spreading by 62%.
According to the Melanoma Research Alliance, the number of new invasive melanoma cases diagnosed annually in the US increased by 27% in the 10 years since 2013. Advanced melanoma can be very hard to treat, and survival very much depends on when the cancer is detected.
From diagnosis, the five-year relative survival rate for localized, early melanoma is over 99%; for melanoma that has spread regionally to nearby lymph nodes and vessels (stage III), it’s 68%; and for melanoma that has spread to distant sites like the brain, lungs, and gut (stage IV), it's 30%.
Biopharmaceutical companies Moderna, Inc. and Merck – known as MSD outside of the US and Canada – have announced the results of Phase IIb of the KEYNOTE-942 study, a clinical trial evaluating the longer-term effectiveness of combining a new melanoma vaccine with an existing anti-cancer immunotherapy drug.
The vaccine, developed by Moderna and called mRNA-4157 (V940), was given in combination with Keytruda (pembrolizumab), Merck’s anti-PD-1 therapy, to 157 patients with high-risk (stage III-IV) melanoma that had been surgically removed. Both boost the immune system’s cancer-fighting abilities. The vaccine is an individualized neoantigen therapy (INT), meaning that it uses the unique DNA signature of a person’s tumor to create a treatment that helps their immune system produce an anti-tumor response. Keytruda blocks the PD-1 pathway that cancer uses to hide in the body, helping the immune system detect and fight cancer cells.
Individualized neoantigen therapies: Exploring one medicine for one patient
After a follow-up of almost three years (median follow-up 34.9 months), the combination treatment reduced the risk of cancer recurrence or death by 49%, compared to treatment with Keytruda alone. Further, compared to Keytruda alone, treatment with both drugs reduced the risk of developing distant metastases or death by 62%.
“The sustained improvements in recurrence-free survival and distant metastasis-free survival observed at approximately three years in the KEYNOTE-942/mRNA-4157-P201 study provide further support of the potential of mRNA-4157 (V940) in combination with Keytruda to help patients with resected high-risk melanoma,” said Dr. Marjorie Green, senior vice president and head of oncology, global clinical development at Merck. “We look forward to building on our legacy of turning breakthrough science into medicines that may have a meaningful impact on patient’s lives as we continue advancing our broad clinical development program evaluating this novel approach with Moderna.”
The most common adverse effects attributed to the combination of mRNA-4157 (V940) and Keytruda were fatigue (60.6%), injection site pain (56.7%), and chills (49%).
The current Phase IIb trial could be considered a ‘mini-Phase III’ trial designed to provide data on the treatment’s effectiveness. Merck and Moderna have now commenced Phase III randomized clinical trials proper to evaluate the mRNA-4157 (V940) vaccine in combination with Keytruda in patients with resected high-risk melanoma and non-small cell lung cancer, the most common type of lung cancer. The companies also, in 2024, began Phase II trials of the combination treatment in patients with renal cell carcinoma, a type of kidney cancer, and bladder cancer.
The researchers presented their trial results at the 2024 American Society of Oncology (ASCO) annual scientific meeting, currently being held in Chicago.
No comments:
Post a Comment