
The theory thus tracks the empiracal but will miss oddities and those come out later like this. It is not the other way around. So we have this to ponder. The theory has to produce nuclear physics.
In the meantime we use a now unlikely but valuable mineral
Lithium, the Elemental Rebel
What a missing element can teach us about the universe.
BY PAUL M. SUTTER
April 10, 2024
https://nautil.us/lithium-the-elemental-rebel-542339/?
Inside every rechargeable battery—in electric cars and phones and robot vacuums—lurks a cosmic mystery. The lithium that we use to power much of our lives these days is so common as to seem almost prosaic. But this element turns out to be a wild card, a rebel that’s been challenging our most basic understanding of the formation of the universe itself.
Beyond the lithium ion-powered batteries, beyond the glass and ceramic manufacture, optical systems, air purification, fireworks and rocket propellants, nuclear weapons, and mood stabilizing pills, lithium is cast about the cosmos. But there is not nearly as much of it out there as there should be. And we don’t know why.
This wily little element has defied explanation for generations, refusing to obey the rest of our cosmological orthodoxy. The robust Big Bang theory, among other accomplishments, allows us to precisely predict the abundances of all of the light elements across the universe.
Except lithium.
Which means there might be something wrong with our understanding of the Big Bang. There might be something wrong with our measurements. There might be something wrong with both. Or this might be a signal that there are new, as-yet undiscovered forces that were at work in the early universe. Whatever the solution is, this rebel and its so-called “cosmological lithium problem” are here to teach us a radical new fact about the universe.
We just have to figure it out.
Here on Earth, lithium had laid underfoot since the planet’s formation, with nobody suspecting that the element even existed. Taken from the Greek word for “rock,” on Earth, lithium is usually only found in trace amounts in larger mineral conglomerations. In 1800, the Brazilian chemist José Bonifácio de Andrada e Silva discovered it as a new ore on the island of Uto, Sweden. Seventeen years later, the chemist Jöns Jakob Berzelius isolated the new element within the ore. Since then, the silvery-white metal has found itself making possible so many of our contemporary luxuries.
But most of the universe’s lithium is bound up inside of stars.
There is not nearly as much lithium in the universe as there should be. And we don’t know why.
Roughly 25 percent of all the lithium in the universe—including the lithium in household electronics—originated in the first few minutes of the Big Bang.
The Big Bang theory tells us that 13.77 billion years ago the entire observable cosmos—containing every star and every galaxy in a volume now spanning more than 90 billion light-years across—was compressed into a volume no bigger than a peach.
Mere moments into the Big Bang, the temperatures were so high that all protons and neutrons were just melted together into their constituent components, which are tiny particles known as quarks. Any time some quarks got together to make a proton, they just got smashed apart by some violent collision or high-energy radiation. But as the universe expanded, it cooled. At a certain point, somewhere about three minutes into the Big Bang, that exotic plasma cooled to around 1 billion Kelvin—still terrifically hot, but cool enough to allow for the production of stable protons and neutrons.
These nuclear reactions continued, with the newly formed protons and neutrons themselves binding together, creating the four lightest elements on the periodic table: hydrogen, helium, lithium, and beryllium. But after about 10-20 minutes of this dance, the expanding universe got too cold to sustain further reactions, locking these elements in place.
It would take until the formation of the first stars, hundreds of millions of years later, for new elements to be fashioned again in the universe
.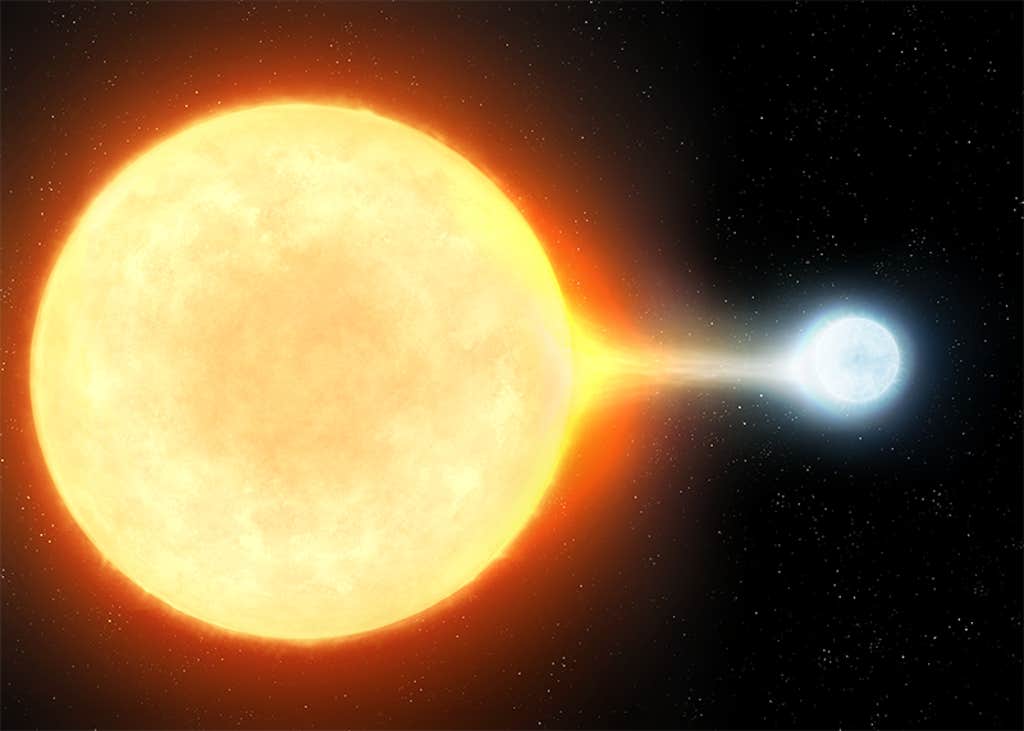

THE BIG PULL: Cosmologists continue to puzzle over the apparent lack of lithium in the universe. They currently think about three quarters of it comes from orbiting star systems in which a dense white dwarf pulls hydrogen off of its partner, eventually sparking a classical nova and a fusion reaction that solders components of hydrogen into lithium. Image by Nazarii_Neshcherenskyi / Shutterstock.
One of the greatest triumphs of the Big Bang theory is its ability to predict the abundance of these light elements. First calculated by physicist Ralph Alpher in the 1940s using the then-new knowledge of nuclear physics, the work was fully fleshed out in a famous paper he coauthored.
These calculations, collectively known as Big Bang nucleosynthesis, offer one of the most powerful predictions of the Big Bang theory: a precise, on-the-nose estimate of how much of the light elements were made in those first fiery minutes, tested by astronomers through the study of the oldest stars, galaxies, and nebulas in the cosmos.
Except lithium.
These nucleosynthesis calculations predict roughly three times as much lithium as has been observed in old stars (our best proxy for early conditions of the universe). Conversely, in keeping with the element’s paradoxical nature, newborn stars tend to have much more lithium than we would expect.
And all of this is only the first quarter of the lithium in the universe. Cosmologists now think the other 75 percent may come from a particular kind of exploding star known as a classical nova. In a classical nova, a white dwarf—the leftover remnant core of a sun-like star—has an orbiting companion. The gravity of the white dwarf vacuums hydrogen gas off of the companion, forming an atmosphere around itself. But when the densities reach a critical trigger point, the hydrogen spontaneously undergoes a runaway fusion reaction, blowing up the atmosphere (and occasionally appearing in our sky as a “new” star—a nova). Because lithium is composed of three protons and some neutrons, the fusion of hydrogen during a nova readily makes lithium in abundance.
Roughly 25 percent of all the lithium in the universe originated in the first few minutes of the Big Bang.
But lithium is also highly unstable, as it has the lowest binding energy of the light elements, meaning that it takes the least amount of energy to tear it apart again. So most of the lithium produced during a nova evaporates into hydrogen and helium. What remains is subject to the ravages of cosmic rays, which can pierce its nuclear heart and destabilize it, vaporizing it in the same way … or be spontaneously created when a cosmic ray strikes a heavier atom.
Confused? You’re not alone. Astronomers cannot figure out which processes play the most important role and what the final tally of lithium should be after all this astrophysical mixing and mashing. All this means that we only have a hazy connection between the lithium produced in the fires of the Big Bang and the lithium that we see scattered across the cosmos today.
When mysteries like this persist through the decades, scientists take it as a sign that the universe is telling them something important. The nature of that important thing is a matter of some debate. And as is usual in physics debates, the theorists blame the observers, and the observers blame the theorists.
One possibility for the strangeness of lithium is that our theoretical understanding of nuclear physics, especially the intense physics operating in the first few minutes of the Big Bang, is not up to par.
This is difficult to reconcile because, given our mastery of nuclear power plants and nuclear weapons, we seem to have a pretty good handle on these interactions. But there are some intriguing openings. For example, extra-rare reaction chains may contribute to some extra lithium without upsetting the balance of the other elements. Or resonances may play a role. These are special combinations of pressure, temperature, and interaction volume that lead to greater-than-average production of elements. We may have underestimated the importance of some of these resonances in Big Bang physics simply because they don’t crop up often in Earthbound experiments.
On the other hand, our observations may be leading us astray. Lithium is a fragile element, so it’s difficult to get a handle on primordial populations that have not been touched or contaminated by other processes. The best we can do is search for the oldest existing stars in the galaxy and try to measure the amount of lithium on their surfaces. These old stars are the most likely to be “pure”—to reflect the primordial amounts of lithium manufactured in the Big Bang rather than in later stellar fusion processes. These measurements are less than straightforward, requiring a significant amount of calibration and fine-tuning, because we can only infer the amount of lithium once we know the star’s temperature and abundance of other elements. If those calibrations are off, so are our estimates of lithium, giving us only a rough and unrefined picture of how much lithium was kicking around the universe when those stars first formed.
As is usual in physics debates, the theorists blame the observers, and the observers blame the theorists.
And it turns out that the surfaces of stars might not even be the best place to look for lithium. The vast bulk of every star is made of the two lightest elements, hydrogen and helium. Lithium, being heavier than both, might simply slink into the inner depths of each star, hiding itself from our view. If it sinks too far down, the intense temperatures and pressures may disassociate it, converting it into even more hydrogen and helium.
The most thrilling possibility is that both the theorists and observers are right, but we’re completely missing some interesting bit of new physics in the early universe. Perhaps some dark matter, the ever-present mysterious particle that appears to make up most of the matter in the universe, can spontaneously decay into other particles, which messes up the nuclear chain reactions of nucleosynthesis and alters the abundances of lithium. Some physicists have even proposed that the fundamental constants of nature, including the speed of light or the charge on the electron, might have been different in the deep and distant past, which would throw off many of our calculations.
While most astronomers think that the answer is likely in some ho-hum direction like the destruction of lithium in stellar atmospheres, that solution has not been confirmed. We can’t crack open stars and see what’s going on inside, and so we can’t directly test the hypothesis.
Some mysteries lead to vast upheavals of previous norms. Some resolve quietly. But no matter what, rebels can be some of the best teachers, if we are open to them. They force us to reconsider the status quo and realign our perspectives. They act as agents provocateurs that guard us from complacency and keep us on our toes. Lithium is doing all that. No scientific theory is perfect, and the Big Bang is no exception. It’s only through the corners, the edges, and the gray areas that we have the opportunity to grow and expand our knowledge of the universe.
The Big Bang theory remains today as the dominant paradigm in physical cosmology. It can explain why the universe is expanding. It can explain the existence of ancient relic radiation. And it can explain the composition and arrangement of matter throughout the cosmos.
Except lithium.
No comments:
Post a Comment