They went on to show that at higher temperatures and pressures, that Xe combines with iron and Nickle as well. This provides a natural Xenon sink for all planets.
This led to a new understanding of what takes place with the Core.
It has opened up an explanation for the hollow Earth that works with classical physics. This is welcome.
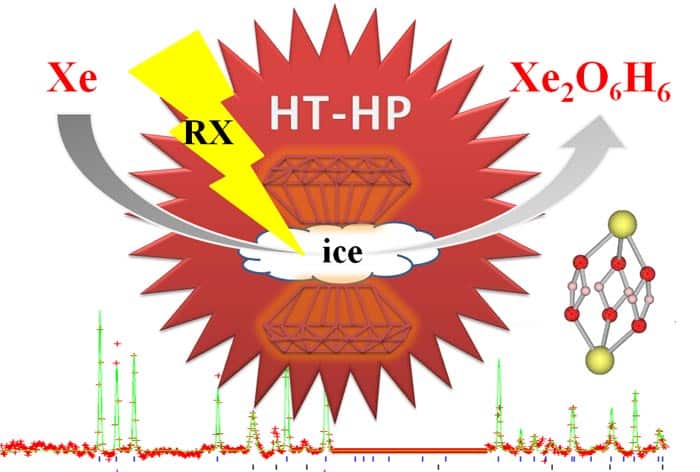
Xenon forms compound at extreme temperature and pressure
The noble gas xenon reacts with water ice at exceedingly high
temperatures and pressures – conditions that are found within the
interiors of planets such as Uranus and Neptune. That’s the conclusion
of an international team of researchers who used X-ray diffraction and
theoretical calculations to determine the crystal structure of the
resulting compound, which has a Xe4O12H12
primitive cell. The findings take planetary scientists one step closer
to solving the “missing xenon paradox” and could also improve our
understanding of xenon’s isotopes.
In the early 1970s researchers noted the surprising lack of xenon
in Earth’s atmosphere when compared with the concentration of other
noble gases – almost 90% of the expected amount of xenon is missing.
However, xenon is found at the expected abundance elsewhere in the solar
system – on meteorites, for example. This suggests that the
mysteriously missing noble gas is merely hiding somewhere on our planet.
Curiouser and curiouser
A multitude of theories have been purported suggesting that, for
example, xenon might have been ejected into space, or be trapped on
Earth in the polar caps, or stuck in sediments, deep in oceanic trenches
or even within the Earth’s core. But none of the theories could account
for all of the missing gas. Further research has also found that both
Mars and Jupiter seem to have a similar lack of xenon within their
atmospheres.
As a noble gas, xenon is assumed to be non-reactive under normal
conditions. But over the years, researchers have tried to make chemical
compounds containing xenon at extreme pressures and temperatures similar
to those that are found deep within the Earth. In 1997 scientists tried
to react xenon with iron under such conditions, but found that no
compound was formed. In 2005 Chrystele Sanloup of Pierre and Marie Curie
University in Paris, along with colleagues, found that the gas could
displace and then substitute silicon in quartz at high temperatures and
pressures. However, the researchers also noted that the xenon escapes
just as easily from the material. Further work, carried out by another
group, found that xenon could also bond to oxygen within quartz,
allowing the researchers to synthesize xenon dioxide (XeO2) for the first time.
Extreme measures
Sanloup (now at the University of Edinburgh) and colleagues in
France, the UK and US have now shown that at pressures above 50 GPa and a
temperature of 1500 K, xenon reacts with water ice to form Xe4O12H12.
Sanloup used a diamond-anvil cell – a device that squeezes a sample
between two tiny, gem-grade diamond crystals. This was laser heated to
create extreme conditions similar to those in the interior of the
“ice-giant” planets Uranus and Neptune. Currently, the atmospheres of
Uranus and Neptune have not yet been probed for their xenon
concentrations.
Sanloup carried out her experiments on the ID27 beam line of the
European Synchrotron Radiation Facility (ESRF) in Grenoble, France.
“Once we were above 50 GPa, we could see a reaction systematically
taking place and could see a distinct diffraction pattern that suggested
a new phase [was formed],” she says. Sanloup went on to tell physicsworld.com
that the structure that best describes the new phase is a hexagonal
lattice with four xenon atoms per unit cell. However, there were several
possible distributions of the oxygen atoms.
Atom build-up
Sanloup then roped in Stanimir Bonev from the Lawrence Livermore
National Laboratory in the US to analyse the structure and resolve the
location of the oxygen atoms. It was during this analytical work that
the team found that none of the solutions with only oxygen worked, so
hydrogen was added to “build up” the final Xe4O12H12
structure. “The hydrogen would not have been seen with the X-ray
diffraction as it would react too lightly,” explains Sanloup. “But in
the future we could use Raman spectroscopy to see it or we could use
neutron diffraction instead of X-rays, but for that we would need a much
larger sample,” she says. The team suggests that its newly discovered
compound has a weakly metallic character and could be formed in
superionic ice – a phase of water that is believed to exist at high
pressures and temperatures.
Sanloup explains that solving the mystery of the missing xenon is
crucial because the relative abundances of radioactive xenon isotopes
are widely used by geochemists as a tool to probe major terrestrial
processes, such as when the Earth’s atmosphere formed. Naturally
occurring xenon has eight stable isotopes and more than 40 unstable
isotopes that undergo radioactive decay. Isotope ratios are also used to
study the early history of our solar system, including to model planet
formation. But most of these calculations assume that xenon is mostly
non-reactive. The new findings could alter our knowledge of the xenon
isotopes, which in turn would affect our models of planet formation and
evolution.

No comments:
Post a Comment