
This is exciting because it can allow us to operate in submarines and space ships way easier. Then a simple heat source can eject the co2 out of the hull and repeat.
Present systems are way messier and likely bulky.
We still need to consume the CO2 so otherwise, not too helpful. grow more trees.
This Yellow Powder Made at UC Berkeley Can Suck Carbon From the Air
Oct 23
https://www.kqed.org/science/1994906/uc-berkeley-scientists-create-turmeric-like-powder-suck-carbon-from-air
UC Berkeley scientists say roughly half a pound of this yellow material can remove as much carbon dioxide from the atmosphere as a tree annually. (Courtesy Zihui Zhou)
Removing carbon dioxide from the air has become an essential part of the world’s battle against human-caused climate change. California alone will need to capture or remove about 100 million tons of carbon dioxide each year — roughly equivalent to the pollution created by 250 gas-power plants — to meet its ambitious climate goals, which include becoming carbon-neutral by 2045.
While some of that can be done through natural means, such as restoring wetlands, technology must fill the gap where natural systems fall short. Scientists at UC Berkeley have developed a material they believe will greatly improve direct air capture — the term for removing carbon dioxide from the air at any location, as opposed to carbon capture, which focuses directly on sources of pollution like smokestacks.
The new material, which is a deep yellow powder that almost resembles ground turmeric, is called a covalent organic framework (COF). COFs have existed for nearly 20 years, but the latest spin on them has excited chemists. The version created at Berkeley can pull relatively large amounts of carbon dioxide from the ambient air with what scientists believe to be incredible effectiveness.
“It’s basically the best material out there for direct air capture,” said Omar Yaghi, a chemistry professor at UC Berkeley and senior author of a paper published Wednesday in the journal Nature about the new COF.
A bit less than half a pound of the stuff can remove about as much carbon dioxide annually as a tree, according to UC Berkeley graduate student Zihui Zhou, who is the first author on the paper.
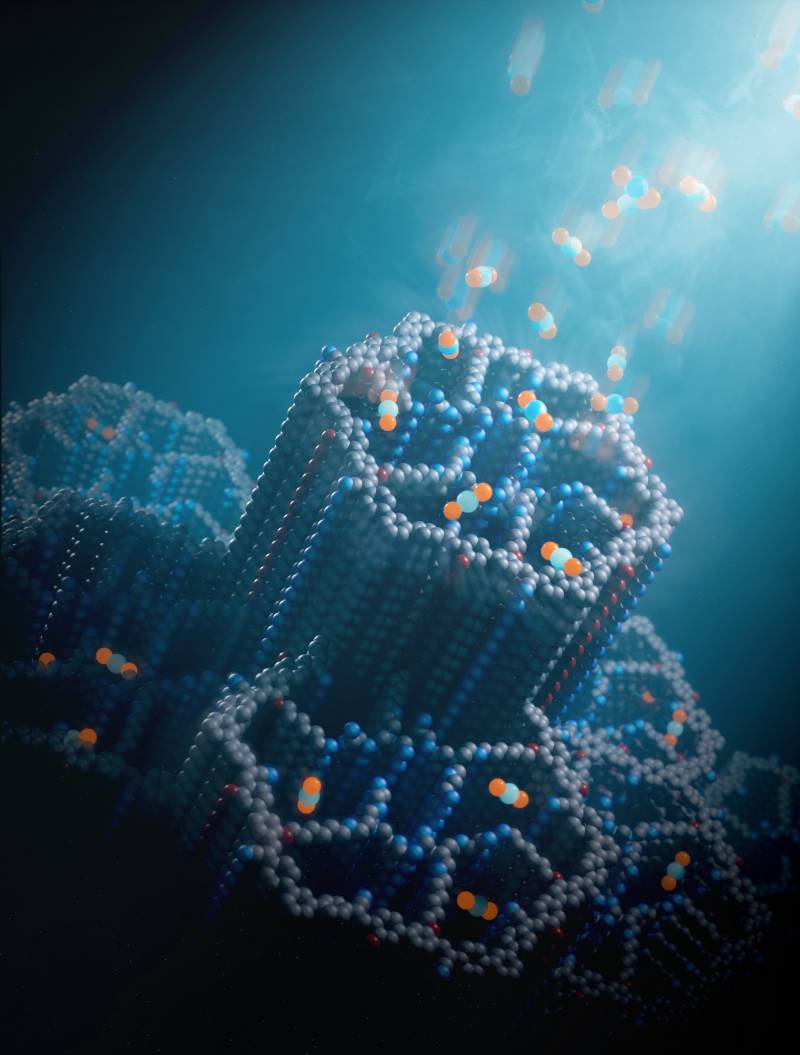
An illustration of an atomic-level look at the new material. The hexagonal channels make the material porous. They are coated with polyamines, which bind with carbon dioxide molecules, as shown by the blue and orange balls. (Courtesy Chaoyang Zhao)
The material works similar to a sponge: When exposed to air, it sucks up carbon dioxide until it is saturated. It’s essentially then wrung out, with scientists capturing that carbon dioxide and storing it away for a significant amount of time, ideally thousands of years. Then, the material is deployed again to absorb more carbon dioxide.
In laboratory tests, Zhou said the team filled a small column about the size of a drinking straw with the COF. They used a pump to push outdoor air through the column until the material was saturated with carbon dioxide.
“It cleaned the air entirely of CO2. Everything,” Yaghi said.
The team then heated the tube to 140 degrees Fahrenheit, which released the gas. They could then use the same material and tube to repeat the process.
The material stands out from previous iterations because it did not degrade when it came into contact with water (or humidity in the air) and remained structurally stable even after 100 uses.
Older versions of COFs absorbed carbon dioxide well from the air that was very saturated with it, like exhaust from power plants, but not well from the ambient air, where concentrations are lower. This new, porous material is lined with molecules called amines that act like microscopic magnets, drawing carbon dioxide to them from air anywhere.
“It’s always exciting when new materials are developed with increased CO2 capacities,” said Jennifer Wilcox, chemical engineering and energy policy professor at the University of Pennsylvania, who was impressed by how the material performed when exposed to water, that it did not use any critical minerals, and required a relatively low temperature to release carbon dioxide. But, she said, “how does this material perform in a system, in a process?”
Wilcox, who was not involved in the research, said half of the equation is the engineering of how to use the high-performing material. “Ultimately, we have to scale these materials up to capture significant amounts of carbon dioxide,” she said. “And when you start to scale them up, you have to think about what the system looks like.”
Zhou and colleagues are working on models of that system right now. One version is a roughly 6.5-foot cube that would be stacked with layers of the COF and tubing for water. Wind or fans would blow ambient air through the box.
The water tubes would control the temperature inside. Once the material inside is saturated with carbon dioxide, engineers warm the box and vacuum out the gas, readying the cube to absorb more of the gas from the air.
“Currently, there are lots of colleagues from engineering departments that have already designed the whole process,” Zhou said. He is hopeful that he and colleagues can simply sub in the new material and that it could be ready for deployment at a larger scale in 1–2 years.
Wilcox, in her own right, has seen similarly quick deployments of new technologies in the past.
No comments:
Post a Comment