understand that if a million folks died,then it is plausible that the majority died because of what appears to be deeliberate manipulation or deliberate omission. the clear alternatives were there and in their face.
ignorance that the gun was loaded does not give you a pass when you point and shoot.
Again ,real science is now coming out and the public will know.
How Ivermectin Trials Were Designed to Fail
Despite the proven efficacy of ivermectin in treating COVID-19, some studies in top-tier journals have concluded otherwise. Which data should we trust?
(eloresnorwood/Shutterstock
By Yuhong Dong, M.D., Ph.D.
4/10/2024Updated:
https://www.theepochtimes.com/health/how-ivermectin-trials-were-designed-to-fail-5616759
Health Viewpoints
The use of ivermectin to treat COVID-19 is an ongoing debate. The central conflict is that although many doctors have reported success in using ivermectin, some studies published in major journals suggest it is in fact ineffective.
Even as the FDA recently has been removing misinformation it posted about ivermectin, the agency has maintained its original position regarding its effectiveness, namely that there isn’t evidence.
People who trust ivermectin claim that the studies showing ineffectiveness are fraudulent, while people who are skeptical of its use for treating COVID-19 view that as an anti-science conspiracy theory.
As a professional with decades of research experience conducting dozens of clinical trials on antiviral drugs, I decided to dive deep into the studies purporting ivermectin’s ineffectiveness. What I found shocked me.
Legacy Media Report IneffectivenessNumerous preclinical studies have found that ivermectin has a broad range of effects on COVID-19, spanning from its initial effect on viral infection to its effect on the pathological changes the virus causes in our bodies.
Ivermectin inhibits the entire life cycle of SARS-CoV-2 in our cells, including attachment, spreading, and replication (1, 2, 3).
Moreover, ivermectin is anti-inflammatory and organ-protective, which can potentially protect against severe COVID-related lung damage and acute respiratory distress syndrome, heart-related complications, and blood clots.
Ivermectin exceeds the approved antiviral effects of other medications, including Paxlovid, molnupiravir, and remdesivir, which target only the virus and lack anti-inflammatory and organ-protective effects. Monoclonal antibodies have to be constructed specific to each variant and are very expensive.
In the pharmaceutical industry, clinical trials are commonly used to evaluate the efficacy and safety of drugs once their mechanism is demonstrated. There are two types of clinical trials: observational and interventional.
Observational studies are often conducted by doctors in clinical, hospital, or community settings to analyze the effects of drugs. The data are collected as observed in clinical practice with minimal interference.
Many doctors have observed the positive effects of ivermectin on their patients. An observational study conducted in Brazil with more than 88,000 patients showed that ivermectin reduced the rates of infection, mortality, and hospitalization by 49 percent, 92 percent, and 100 percent, respectively.
Pharmaceutical companies are required to conduct interventional studies that meet the approval standards set by the U.S. Food and Drug Administration (FDA). Randomized clinical trials (RCTs) are frequently used to fulfill these requirements. This type of study is considered the gold standard and involves randomly assigning one group of patients to receive a specific drug while the other group does not receive it, then comparing the outcomes.
Legally and medically, ivermectin can be prescribed off-label to treat COVID-19 since it has already been approved by the FDA for other diseases.
Although many doctors have observed the positive effects of ivermectin in treating their patients, the media have specifically highlighted data from a few selected RCTs that have concluded it is ineffective in treating COVID-19.
However, some critical aspects were overlooked in those RCTs.
Click here to watch the full documentary “The Unseen Crisis: Vaccine Stories You Were Never Told”
Improper DosingA drug’s therapeutic effects can be observed only when it reaches the appropriate concentration in the body and remains there for a few days, allowing sufficient time to work.
Improper dosing was a major issue in the RCTs that found ivermectin ineffective.
trtfg
Recommended DosageAccording to Merck’s package insert for ivermectin (brand name Stromectol), a single oral dose of 0.2 mg/kg was officially recommended for treating parasitic diseases. There is no official dose for COVID-19.
The recommended dosage of ivermectin for treating COVID-19 is based on the clinical experiences of physicians worldwide.
The Front Line COVID-19 Critical Care Alliance (FLCCC) guidelines recommend taking 0.4 mg/kg of ivermectin daily, immediately after exposure. Once a cumulative dose in excess of 200 mg is reached, the risk of acquiring COVID-19 has been shown to be nearly zero.
It is common for a drug with multiple indications to have different doses for different diseases.
Moreover, ivermectin should be given with food, as it has a 2.6-fold higher bioavailability when taken with food than when taken on an empty stomach. The Merck package insert (revised May 2022) also supports this and states, “Administration of 30 mg ivermectin following a high-fat meal resulted in an approximate 2.5-fold increase in bioavailability relative to administration of 30 mg ivermectin in the fasted state.”
FLCCC guidelines also recommend taking ivermectin “with or just following a meal for greater absorption.”
Yet this important dosing information is not reflected in the commonly used drug prescribing resource known as the Prescribers’ Digital Reference, which states: “Take the number of tablets your doctor has prescribed all at the same time with water on an empty stomach. Do not eat any food within two hours before or after taking the tablets.”
So if a person takes the dose while fasting, they are getting only 40 percent of the recommended dose. For patients with a higher body weight, the effects of underdosing could be even more significant.
RCT Studies Used Inappropriate DosingIn the most recent PRINCIPLE trial published in March, ivermectin was used at 0.3 mg/kg for only three days. Moreover, it was designed to dose the ivermectin without food: “Participants were advised not to eat two hours before or after taking ivermectin.”
In another RCT, ACTIV-6, published in JAMA in October 2022, ivermectin was dosed in a fasting status; the protocol stated, “Ivermectin should be taken on an empty stomach with water (30 minutes before a meal or 2 hours after a meal).”
Ivermectin was reported as dosed at 0.4 mg/kg for three days—a much shorter time period than it should be. However, in the protocol Table 4 in Appendix 16.3.3, the precise dosing was as low as 0.269 mg/kg, and 0.4 mg/kg is actually only the upper dose limit—not the real dose.
According to the worldwide recognized study guideline ICH Good Clinical Practice, clinical trials must adhere to ethical principles. Failure to do so would be considered study misconduct or fraud and would violate the principle of integrity.
Another JAMA study published in March 2021 repeated the same mistake in mild COVID-19 patients by suggesting they take 0.3 mg/kg for five days on an empty stomach.
An RCT study known as TOGETHER, published in March 2022 in the New England Journal of Medicine, underdosed ivermectin with 0.4 mg/kg for only three days and did not mention dosing with food.
Nevertheless, even at this low dose, the ivermectin reduced hospitalization rates, death, and the need for mechanical ventilation compared to a placebo.
Clinical Improvement Despite UnderdosingIt is inappropriate to conclude that ivermectin was ineffective based on these RCT studies with major design flaws.
Despite the poor study design, ivermectin showed clinical benefits and saved lives.
In the PRINCIPLE study, self-reported recovery was shorter in the ivermectin group than in the group receiving usual care, with a median decrease of 2.06 days. The statistical analysis showed that it met the predefined superiority criteria.
Furthermore, the analysis showed that ivermectin effectively reduced COVID-19-related hospitalizations and deaths. Only 1.6 percent of 2,157 patients in the ivermectin group experienced hospitalizations or deaths, compared with 4.4 percent of 3,256 patients in the usual care group.
Even a low dose of ivermectin has demonstrated the potential to save lives. However, the authors concluded, “Ivermectin for COVID-19 is unlikely to provide clinically meaningful improvement in recovery, hospital admissions, or longer-term outcomes.”
Meanwhile, the report’s appendix includes dozens of recorded clinical benefits in patients treated with ivermectin, such as the time it took to alleviate all symptoms, general unwellness, muscle aches, and headaches. The improvement of symptoms was also sustained, and the severity was reduced. Surprisingly, the source PDF was removed from the website during the writing of this article.
There are additional examples. Although the previously mentioned 2021 JAMA study underdosed patients, treatment with ivermectin reduced recovery time by two days. In the ACTIV-6 study, only one venous blood clot event was reported in 817 ivermectin-treated patients, compared with five events in 774 placebo-treated patients.
Statistical FailuresIt is important to note that the definition of treatment effects in an RCT can differ from those discussed in real-life observational studies.
Sometimes, even if the results of a clinical trial demonstrate a clear effect, the conclusion may still be interpreted as ineffective because of the statistical definition of effectiveness.
Interpreting statistics can be challenging as they usually involve complicated mathematical models and numerical data that can be manipulated to support a specific agenda. Nevertheless, for the purpose of this discussion, let’s presume that all research is carried out conscientiously and without manipulative intent.
In a randomized, double-blind, placebo-controlled clinical trial with mild to moderate COVID-19 patients, none of the 55 patients in the ivermectin group died, whereas four of 57 in the placebo group died. Moreover, only 1.8 percent of ivermectin-treated patients needed invasive ventilation compared with 8.8 percent in the placebo group.
In other words, ivermectin reduced the risk of death by 100 percent and the need for ventilators by 80 percent.
However, the article did not provide the p-value (probability value) for the death rate comparison or the invasive ventilation of 0.102 (Table 2), which is higher than the 0.05 threshold considered to be a significant statistical difference.
P-values are commonly used to test and measure a “null hypothesis,” which states that no differences exist in the effects being studied between two groups. A finding is considered statistically significant and warrants publication when the p-value is 0.05 or less.
The p-values in this study were deemed insignificant because they were more than 0.05. Accordingly, the authors wrote that this difference was statistically insignificant and concluded that ivermectin “had shown only marginal benefit.”
How could a 100 percent reduction in death or an 80 percent reduction in ventilation be interpreted as “marginal” effects?
In the I-TECH study published in JAMA Internal Medicine in 2022, the patients treated with ivermectin had a mortality rate of 1.2 percent compared with 4 percent in the comparator group.
The same conclusion was made as the previous study because the p-value was 0.09, higher than 0.05.
If the 7 million patients reported to have died from COVID-19 had been treated with ivermectin, an estimated 4.9 million lives could potentially have been saved based on the 70 percent reduced mortality rate from the I-TECH study; or 4.5 million lives could have been saved based on the 64 percent reduction of mortality in the PRINCIPLE study.
The lifesaving potential of ivermectin has been hindered by the unnecessary statistical threshold. The problem of statistical significance is widespread and frequently causes confusion among scientists.
A 2016 Nature article raised concerns about the misuse of p-values. A 2019 comment in the same journal stated, “The misuse of statistical significance has done much harm to the scientific community and those who rely on scientific advice.”
The authors called for abandoning the use of statistical significance to draw conclusions regarding the effectiveness of drugs, such as stating that “drug Y does not work,” and cautioned that such conclusions may result in the dismissal of potentially lifesaving drugs.
The authors also wrote, “Let’s be clear about what must stop; we should never conclude there is ‘no difference’ or ‘no association’ just because a P value is larger than a threshold such as 0.05.”
Selection BiasMany people, including physicians, may not be aware that interventional studies, particularly RCTs, are prone to numerous biases, with selection bias being one of the most significant. Excluding potentially eligible individuals because of their anticipated group allocation can lead to selection bias.
It’s common knowledge that early treatment of COVID-19 is crucial for effective results. The earlier the treatment starts, the more effective it is. These approved antivirals for COVID-19 are used shortly after COVID-19 infection and usually within a few days after symptom onset.
For example, Paxlovid and molnupiravir registration trials treated patients within only five days of symptom onset.
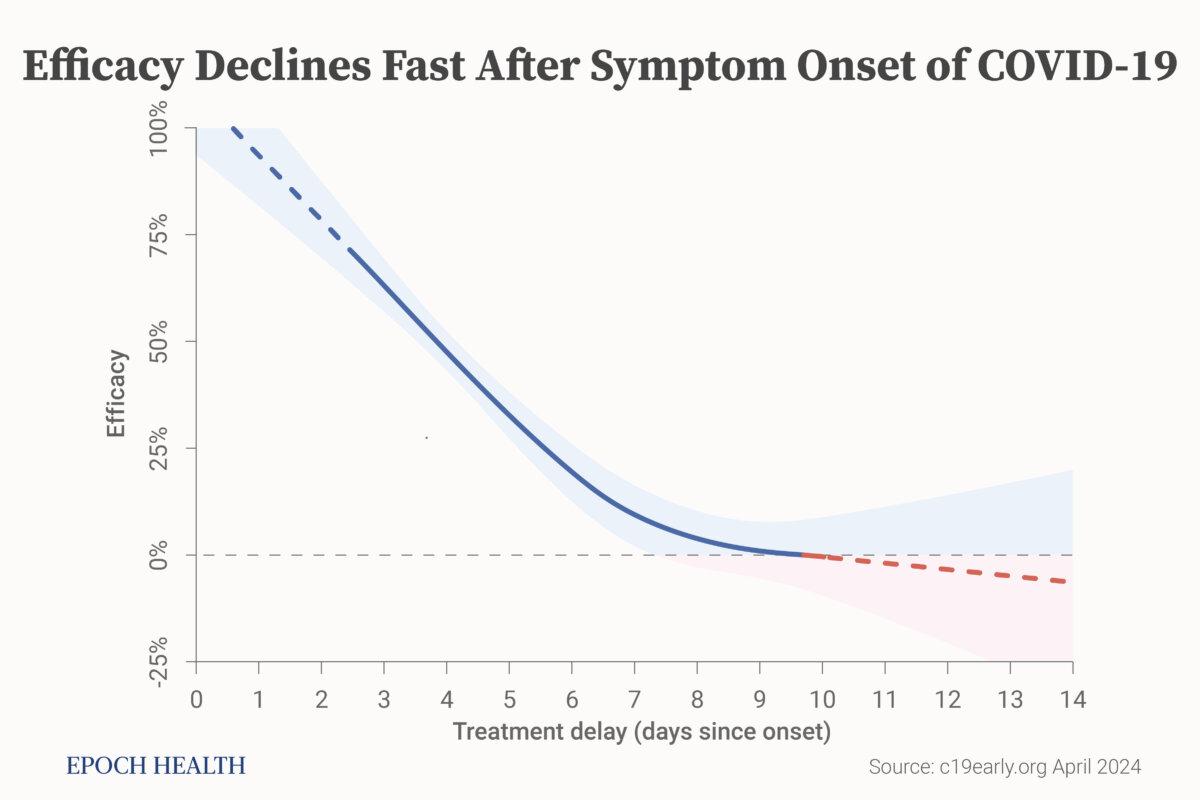
Early treatment is critical for COVID-19. Efficacy declines rapidly with treatment delay. (c19early.com)
However, in the PRINCIPLE trial, ivermectin was used for patients within 14 days of symptom onset, while ACTIV-6 treated patients an average of six days after infection.
Patients with severe kidney disease are normally excluded from phase 3 studies, as they are less likely to respond to antiviral treatment. This approach has been taken by remdesivir (protocol), molnupiravir (protocol), and Paxlovid (protocol). However, such standard exclusion criteria were not taken by the ACTIV-6 or PRINCIPLE study protocols.
Why was ivermectin treated so unfairly in these clinical trials?
It is well known that when an RCT is sponsored by Big Pharma, there is often a financial conflict of interest, as the research institutions are usually hired or funded by the pharmaceutical company. In a world where wealth often competes with ethics, how many can resist financial temptation and stay true to moral principles?
“Hidden agenda bias” occurs when a trial is conducted to demonstrate a desired outcome rather than to answer a question.
Proven Without a Profit MotiveConducting an RCT to get a drug approved by the FDA requires money. Every drug must be managed by a professional team composed of doctors, database managers, and assistants. Professionals must secure funding, recruit a lead investigator, and find hospitals to conduct the study. An operational team must perform the study, analyze the data, and gain FDA approval.
Since ivermectin is a generic drug that lacks profitable marketing and a pharmaceutical sponsor, it’s challenging to organize and systematically manage its new application with health authorities, data, and customers.
Nevertheless, doctors worldwide have been using ivermectin to help patients and have collected valuable data.
The website c19ivm.org has compiled data on 102 clinical trials proving ivermectin’s consistent effectiveness in treating COVID-19. Studies with negative conclusions about ivermectin are also included, such as the four RCTs with recognized design flaws.
Since the beginning of the analysis, ivermectin has consistently shown efficacy. This meta-analysis provides a thorough and transparent real-time analysis of all eligible ivermectin studies.
The trials were conducted by 1,139 doctors or scientists from 29 countries with 142,307 patients. Out of the total studies, 86 have been peer-reviewed with 128,787 patients, and 49 were randomized controlled trials with 16,847 patients.
In the studies with comparative groups, ivermectin was shown to reduce the risk of COVID-19 infection by 81 percent, mortality by 49 percent, ICU admission by 35 percent, ventilation usage by 29 percent, and hospitalization by 34 percent.
In comparison with the control groups, the group treated with ivermectin as a preventive measure before infection experienced reductions in the most severe clinical outcomes of COVID-19 by 85 percent. When used in the early stage of COVID-19, ivermectin decreased the severity of the disease by 62 percent, and when used in late stages, it reduced the clinical severity by 39 percent. Clinical severity is measured by death, ventilation, disease progression, and hospitalization.
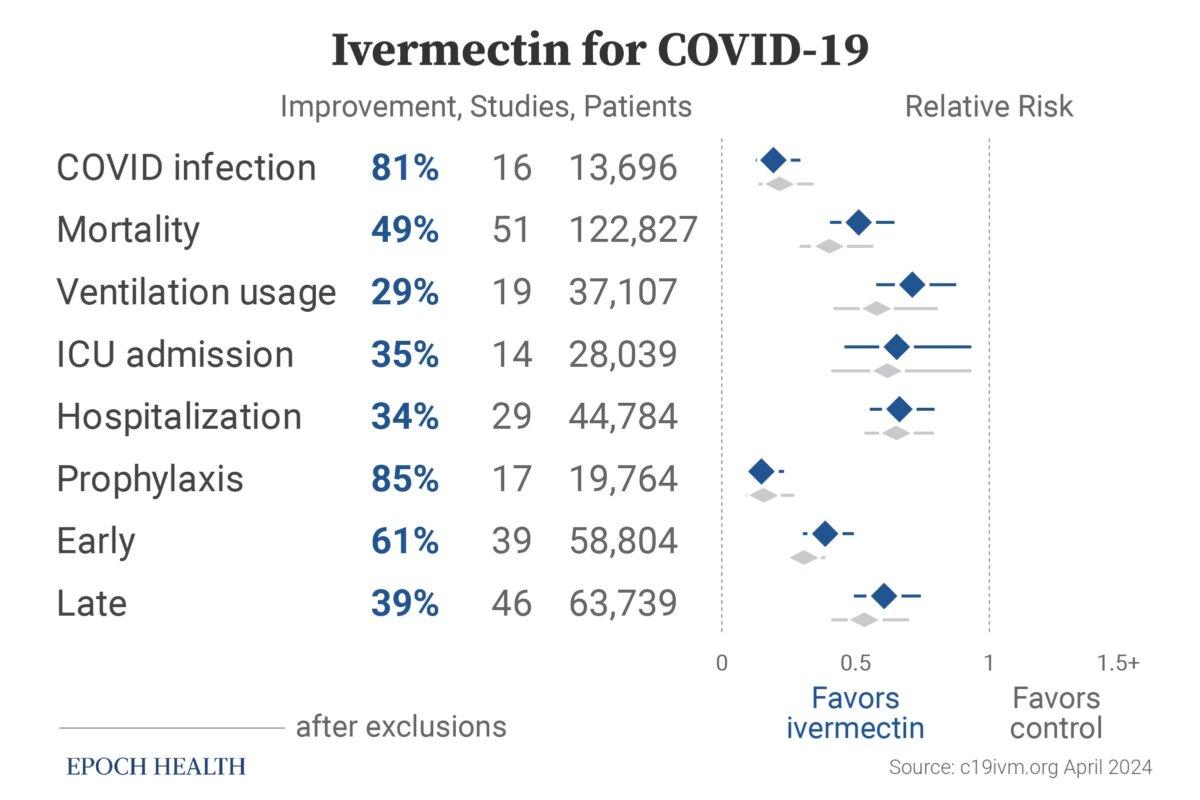
Ivermectin treatment effects in COVID-19 patients, based on a meta-analysis of 102 clinical trials. (c19ivm.org)
Considering the Entire PictureIt’s difficult to believe that the designers of these studies were unaware of the dosing of ivermectin. Despite all the above analyses, the reasoning behind the ivermectin underdosing or unfavorable study design may be linked to factors beyond science.
A new drug or vaccine cannot achieve an emergency use authorization status if there is an existing viable therapeutic available. This fact alone may have affected many decisions.
The NIH website lists only those RCTs that I found to have design flaws (or potential fraud) to justify its recommendation against the use of ivermectin in the treatment of COVID-19.
Peer-reviewed studies showing the efficacy of ivermectin in treating COVID-19 have been retracted without explanation, and doctors have been demonized, censored, and doxxed for speaking the truth.
Legacy media, including The New York Times and CNN, reported incomplete and improperly interpreted trials that failed to present an accurate representation of ivermectin’s effects.
It’s important to keep an open mind and consider the entire picture when examining the ivermectin issue, rather than dismissing it as conspiracy or misinformation. This can lead to more informed decisions that could ultimately save lives.
No comments:
Post a Comment