
At least we have a useful strategy that can work for other meds as well. We need anuy form of progress in mental illness.
Having folks on known nasties until they cook off is hardly to be recommended.
so yes this is promising with the med used and for locating comparables as well.
finally it is a good starting point.
A Vaccine for Depression?
Ketamine’s remarkable effect bolsters a new theory of mental illness.
BY TAYLOR BECK
December 10, 2015
Illustration by Francesco Izzo



A Vaccine for Depression?
Ketamine’s remarkable effect bolsters a new theory of mental illness.
BY TAYLOR BECK
December 10, 2015
Illustration by Francesco Izzo
https://nautil.us/a-vaccine-for-depression-235721/
One sunny day this fall, I caught a glimpse of the new psychiatry. At a mental hospital near Yale University, a depressed patient was being injected with ketamine. For 40 minutes, the drug flowed into her arm, bound for cells in her brain. If it acts as expected, ketamine will become the first drug to quickly stop suicidal drive, with the potential to save many lives. Other studies of ketamine are evaluating its effect as a vaccination against depression and post-traumatic stress. Between them, the goal is nothing less than to redefine our understanding of mental illness itself.
Depression is the most common mental illness in the United States, affecting 30 percent of Americans at some point in their lives. But despite half a century of research, ubiquitous advertising, and blockbuster sales, antidepressant drugs just don’t work very well. They treat depression as if it were caused by a chemical imbalance: Pump in more of one key ingredient, or sop up another, and you will have fixed the problem.

Prepared: One day, soldiers heading into combat could be treated to reduce the chance of getting PTSD.Co Rentmeester/Getty Images
But the correspondence between these chemicals (like serotonin) and depression is relatively weak. An emerging competitive theory, inspired in part by ketamine’s effectiveness, has it that psychiatric disease is less about chemical imbalance than structural changes in the brain—and that a main cause of these changes is psychological stress. “I really do think stress is to mental illness as cigarettes are to heart disease,” says Gerard Sanacora, the psychiatry professor running the ketamine trial at Yale.
The theory describes stress grinding down individual neurons gradually, as storms do roof shingles. This, in turn, changes the nature of their connections to one another and the structure of the brain. Ketamine, along with some similar molecules, acts to strengthen the neuron against that damage, affecting not just the chemistry of the brain but also its structure.
Mental hospitals don’t usually see patients until they break: a brain shaped by vulnerable genes, wrecked by the stress of loss or trauma. This isn’t how it works with other sicknesses: heart disease, cancer, AIDS. Detected early, these conditions can often be managed. Crises averted.
If Sanacora and like-minded researchers are right, we may be on the cusp of a sea change that allows for a similar approach to mental health. The new approaches may prevent mental illness before it hits, by delivering a vaccination for the mind.
The need for progress could hardly be more urgent: Of all illnesses, neuropsychiatric diseases are estimated to put the heaviest burden on society. Nearly half of Americans are affected by some sort of mental disorder at some point in life. Suicides, 90 percent of them among the mentally ill, take 40,000 Americans every year—more than murder or car crashes. Since 2005, the suicide rate among U.S. war veterans has nearly doubled; in the first half of 2012, more service members died by suicide than in combat. Few medical failures are more flagrant than psychiatry’s impotence to save these people.
At the same time, treatment can be woefully ineffective. Less than a third of depression patients respond to a drug within 14 weeks, according to the 2006 STAR*D trial, the largest clinical test of antidepressants. After six months and multiple drugs, only half of patients recovered. Thirty-three percent don’t respond to any drug at all. When the pills do work, they are slow—a deadly risk, given that people with mood disorders kill themselves more often than anyone else.
Our treatments work so poorly in part because we don’t really understand what they do. Serotonin, the most common target for current antidepressants, is a neurotransmitter, a chemical that carries messages in the brain. But it was first found, in 1935, in the gut. Serotonin’s name comes from blood serum, where Cleveland Clinic scientists discovered it in 1948, noting that the chemical helps with clotting.
When Betty Twarog, a 25-year-old Ph.D. student at Harvard, later found serotonin in neurons, she wasn’t taken seriously. At that time, brain signals were thought to be purely electrical impulses that leapt between cells. Twarog called this old idea “sheer intellectual idiocy,” as Gary Greenberg reports in his book Manufacturing Depression. Working at the Cleveland Clinic in 1953, she found serotonin in the brains of rats, dogs, and monkeys.

K: One obstacle to the therapeutic use of ketamine is its reputation as a recreational drug.Wikipedia
Twarog didn’t know yet what serotonin was doing there, but a clue came soon from D.W. Woolley, a biochemist at Rockefeller University, in New York. In 1954 Woolley pointed out in a paper that lysergic acid diethylamide, or LSD, is chemically similar to serotonin and is processed similarly in the brain. Since LSD “calls forth in man mental disturbances resembling those of schizophrenia,” he wrote, another drug affecting serotonin might be used to treat schizophrenia. Twarog’s original paper would take years to percolate through the male-dominated field, but her work and Woolley’s would become accepted as evidence of how important chemicals like serotonin could be to brain signaling. The discovery was a breakthrough for neuroscience—but it also birthed a misleading, long-lived belief about mental illness. “The thesis of this paper,” Woolley wrote, “is that … serotonin has an important role to play in mental processes and that the suppression of its action results in a mental disorder. In other words, it is the lack of serotonin which is the cause of the disorder.”
Around the same time, other researchers stumbled on the first antidepressants, iproniazid and imipramine. Intended to treat tuberculosis and schizophrenia, respectively, these drugs also happened to make some patients “inappropriately happy.” Researchers found that the drugs elevated levels of serotonin, along with related neurotransmitters.1 This began a huge search to find chemically similar drugs that worked better as antidepressants.
Drug companies often say mood disorder is caused by a “chemical imbalance.” But the evidence for this story is slim.
Iproniazid was the first of a class of medicines that block an enzyme from breaking down serotonin, as well as dopamine and norepinephrine, two other neurotransmitters. The chief downside of these drugs, called monoamine oxidase inhibitors (MAOIs), is that they require a strict diet: no aged cheeses, wine, beer, or cured meats. Combined with these foods, the drugs can cause deadly spikes in blood pressure, a hassle that often inclines patients to ditch them. (The novelist David Foster Wallace took an MAOI for decades; in part to escape the food restrictions, he got off the drug months before his suicide.) On the other hand, tricyclic antidepressants, like imipramine, work by blocking the re-absorption of serotonin and norepinephrine. The cost is a host of side effects, from dry mouth to weight gain to erectile dysfunction and loss of libido.
The next generation of drugs focused on fine-tuning the same mechanisms, and had somewhat improved side effects. A new class of drugs known as selective serotonin reuptake inhibitors, or SSRIs, arrived in the ’80s, bringing huge commercial successes like Prozac, Zoloft, and Paxil. Since SSRIs are more specifically focused on serotonin, they were heralded as cleaner options; but they are not much more effective at lifting mood than the older drugs. We often take for granted the diabetes analogy for depression: If you are depressed, it is because you need serotonin, just as a diabetic person needs insulin. Drug companies often say that mood disorder is caused by a “chemical imbalance” in serotonin or a signal like it. One ad for Zoloft, the blockbuster antidepressant, featured a sad white circle crawling cutely beneath a gray cloud; the voice-over boasted that depression may be “related to an imbalance of natural chemicals in the brain. Zoloft works to correct this imbalance.”
But the evidence for this story is slim. Prozac raises serotonin levels within hours yet doesn’t change mood for weeks. When scientists deplete serotonin in healthy people, it does not make them sad. And when doctors measure serotonin levels in the cerebrospinal fluid of depressed people, they do not find a consistent deficiency; one 2008 study even found increased levels of serotonin in depressed people’s brains. The drug tianeptine, discovered in the late ‘80s, decreases serotonin levels yet relieves depression. And studies have shown that people falling in love show lower, not higher, levels of serotonin.
Serotonin is clearly not just a feel-good chemical. If a serotonin-based drug like Zoloft makes you happier, it works in some other, indirect way. As psychiatrist Ronald Pies, editor of Psychiatric Times, put it in 2011, “The ‘chemical imbalance’ notion was always a kind of urban legend—never a theory seriously propounded by well-informed psychiatrists.”
Meanwhile, as serotonin falls far short of explaining depression, a more likely candidate is emerging.
Stress in moderation is not harmful, but motivating. Cortisol, a stress hormone, cycles daily; synchronizing with sunlight, it helps arouse us for the day. In health, the hormone spikes when we need to pay attention: a test, a job interview, a date. Studies on rodents and humans confirm that brief, mild increases in stress are good for the brain, particularly for memory. During these spikes, neurons are born and expand in the hippocampus, the seahorse-shaped finger of tissue responsible for forming new memories and understanding three-dimensional space, and rodents learn better. The student who gets stressed while studying is more alert and remembers more than the one who feels no urgency—up to a point. The problem comes when stress is either too intense at one moment, as in a rape or violent attack, or too sustained, as in long-term poverty, neglect, or abuse.

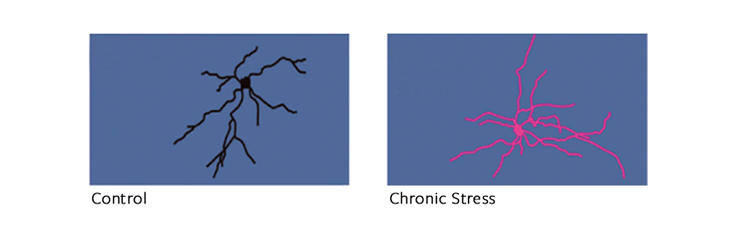
ACCENTUATING THE NEGATIVE …: Under prolonged stress, neurons in the amygdala, the brain’s fear center, expand like overgrown shrubbery and become hyperactive.Image from “Nature Reviews Neuroscience”*
Stress changes brain architecture differently, depending on how long it lasts. After chronic stress, like childhood trauma, the effect of hormones on brain cells inverts: Neurons in the hippocampus and the prefrontal cortex, which is responsible for mood and impulse control, start to shrink, while those in the amygdala, the almond-shaped seat of fear and anxiety, expand like overgrown shrubbery. But people are differently vulnerable, depending on genes and on prior life experience. “If you take two people and subject them to the same stressful event, for one of them it will be harmful and for the other, no,” says Maurizio Popoli, a professor of pharmacology at the University of Milan. “It is because they perceive the stress differently.”

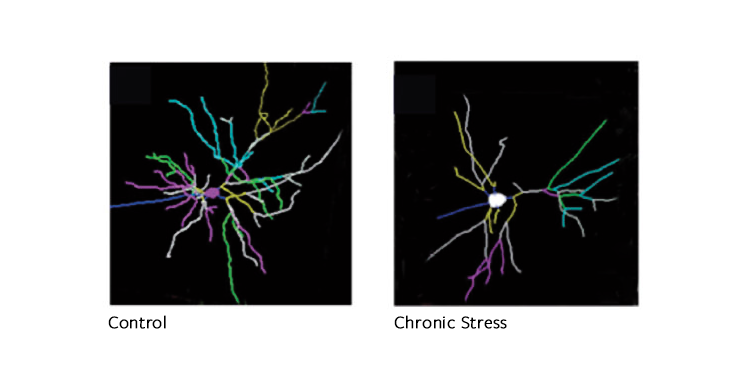
… AND ELIMINATING THE POSITIVE: In the prefrontal cortex and hippocampus, regions responsible for memory, attention, and self-control, chronic stress shrinks the branches of neurons.Image from “Nature Reviews Neuroscience”*
Stress hormones’ most important effect is to flood parts of the brain with glutamate, the brain’s “go” signal. Used by 80 percent of neurons in the cortex, this key neurotransmitter drives mental processes from memory to mood. Glutamate triggers neurons to generate sudden bursts of electricity that release more glutamate, which can in turn trigger electrical bursts in nearby neurons.
This cellular signaling is called excitation and is fundamental to how information is processed in the brain. Like sexual excitability, it ebbs and flows; a “refractory period” follows each neural firing, or spike, during which the neuron cannot be excited. Other neurotransmitters, like serotonin, are called “modulatory,” because they change the sensitivity of neurons that secrete glutamate (among others). Less than 1 percent of neurons in the cortex signal with these modulators. As Popoli puts it, these modulators are “very important for fine-tuning the machine. But the machine itself is an excitatory machine,” driven by glutamate.
Glutamate moves like a ship between neurons. The sea it sails is called the synapse, the shore it departs from is the presynaptic neuron, and the destination, on the synapse’s far side, is the postsynaptic neuron. Another component, called a glial cell, works to remove glutamate ships from the synapse and recycle them. The glutamate system is affected at each of these points by stress hormones: They push the first neuron to send more ships, interfere with the glial cell’s recycling, and block the docks on the distant shore. All of these changes increase the number of glutamate ships left in the synapse, flooding the cell with aberrant signals. Indeed, depressed people’s brains, or at least animal models of depression, show all three of these problems, leading to long-lasting excesses of glutamate in key portions of the brain.
This superabundance of glutamate makes a neuron fire sooner than it should and triggers a cascade of signals inside the cell, damaging its structure. Glutamate binds to the neuron and allows in a flood of positively charged particles, including calcium, which are vital to making a neuron fire. But in excess, calcium activates enzymes that break down the neuron. Each neuron has tree-like branches, called dendrites, which are used to communicate with other neurons. When overdosed in glutamate, this canopy of branches shrinks, like a plant doused with herbicide. First the “twigs,” called spines, disappear. After prolonged stress, whole branches recede.
This harmful process, called excitotoxicity, is thought to be involved in bipolar disorder, depression, epilepsy, and neurodegenerative diseases like Alzheimer’s, Huntington’s, and Parkinson’s. In depressed brains, many areas are shrunken and underactive, including part of the prefrontal cortex and the hippocampus. The brain changes that cause mood disorders, Sanacora and his colleagues believe, come in part from chronic stress overexciting neurons with glutamate.
Ketamine works faster than any other drug, and for up to 65 percent of patients who don’t respond to existing treatments.
We usually think of our brains’ adaptability as a good thing. Just as neurons grow during development, the wiring in the adult brain can change. After strokes or other brain injuries, neural signals re-route themselves around damage, allowing even very old people to re-learn lost skills. Psychotherapy and meditation can change patterns of brain activity in ways that persist after treatment.2
But the neuroplasticity hypothesis of mental disorder highlights the drawback of such neural liberalism: The human brain’s flexibility allows regeneration, but also renders it vulnerable to being altered by stress. Subjected to the trauma of war, a bad breakup, or a bout of homelessness, a person with a genetic predisposition may find his mind stuck in a loop of chronic fear or depression.
The mood drugs in wide use now focus on modulatory neurotransmitters like serotonin. Ketamine, however, works directly on glutamate signaling. If ketamine is tapping into the root of the problem, this might explain why it works faster, better, and more often than more popular antidepressants.
Not everybody accepts the idea that glutamate and stress are central to depression. Some experts see the effects of stress as downstream effects, not the root cause of mood disorder. “The mechanism of action of a good treatment does not have to be the inverse of a disease mechanism,” says Eric Nestler, an expert on addiction and depression at Mt. Sinai Hospital. Serotonin drugs and ketamine may affect depression indirectly, without a serotonin or glutamate abnormality at the root of depression. Nestler also points out that depression probably includes a diversity of subtypes, without any single cause. He treats depression not as a unified disease, but a constellation of symptoms, each with discrete neural roots.
Even so, we do know that ketamine works faster than any other drug, and for up to 65 percent of patients who don’t respond to existing treatments.
If ketamine turns out to be a psychiatric savior, it will be one with a surprising history. Since 1962 it has been a go-to anesthetic for children in emergency rooms, because it kills pain, muffles consciousness, and rarely causes breathing or heart problems. Children given ketamine enter “a trance like state of sensory isolation” free of pain, memory, and awareness, as one review put it. Emergency room doctors rely on ketamine to make sure kids have no awareness or memory of, say, the trauma of having a shattered arm set back into place.
On the other hand, ketamine is a well-known recreational drug with potential for abuse. The dissociative trip caused by a moderate dose of ketamine has made it popular in clubs and raves since the 1970s, especially in Asian cities like Hong Kong. Its sedative effect made “special K” a date-rape drug. Doctors, patients, and the government agencies that fund research are often suspicious of a drug known to cause hallucinations, as they have been of psychedelics like psilocybin and ecstasy, despite their potential for treating depression or anxiety. Each tends to show fast results after a single dose, like ketamine.
In 1999, the same year ketamine was declared a controlled substance in the United States, Yale researchers happened upon its antidepressant power. A team co-led by Dennis Charney, now dean of the Icahn School of Medicine at Mt. Sinai, in Manhattan, and John Krystal, now chair of the department of psychiatry at Yale, used ketamine to study glutamate: Since ketamine was known to block glutamate receptors, it might show what role the excitatory neurotransmitter plays in the depressed brain. To their surprise, they found that the drug made patients feel better, often within hours. A single dose, much smaller than what’s used for anesthesia, tended to last for weeks.
Since 1999, a dozen studies have replicated the results, often on patients who failed to respond to other drugs. Ketamine also works for bipolar people in depressive phases, without triggering mania, as classic antidepressants sometimes do. The majority of depressed people studied have responded to ketamine. For patients who are often suicidal, this fast response can be lifesaving. Some 50 doctors in the U.S. now offer ketamine infusions for depression.
The first evidence in humans that ketamine might work to prevent mood disorder came from the battlefield.
Many leaders in the field see the emergence of ketamine, and future fast antidepressants based on glutamate, as a great leap forward for the field. “In my mind,” Sanacora told NPR recently, “it is the most exciting development in mood-disorder treatment in the last 50 years.”
Ketamine and the old antidepressants both result in fuller neural “trees,” but by different routes, at different speeds. Prozac and other serotonin-based drugs take four to six weeks to kick in. A landmark 2003 Science study by Columbia University’s René Hen and Ronald Duman, now at Yale, found that serotonin-based antidepressants only work if they spur birth of new neurons in the hippocampus.3 These new neurons take four to six weeks to mature, roughly the same amount of time that conventional antidepressants take to lift a depressed person’s spirits. A 2010 paper argued that SSRIs like Prozac may work by dampening glutamate release in response to stress. So even old-school antidepressants, when they work, may act on the glutamate system.
Ketamine, on the other hand, seems to act directly on mature neurons, fertilizing them to grow branches more robustly, or protecting them against damage. Ketamine’s key effect is to block glutamate receptors of one type. This causes less calcium to flow into the neuron, reducing the risk of the neuron shrinking or self-destructing.
Today ketamine is offered by psychiatrists and anesthesiologists, at prices ranging from $300 to $1,000 per dose, for people who are morbidly depressed or have chronic pain. Insurance doesn’t usually cover the cost of an infusion, because even though it is FDA approved as an anesthetic, it has not been approved as an antidepressant. Each new use of a drug requires multiphase clinical trials for FDA approval, usually funded by pharmaceutical companies, which have little incentive to invest in a drug they can’t monetize. Ketamine got its original patent in 1966, and that expired long ago. So even if drug companies steered ketamine through the expensive approval process as an antidepressant, doctors could still prescribe the cheap, generic versions already available for anesthesia instead of pricier, patented versions intended for depression. This is an old story. Lithium carbonate, which also acts on glutamate receptors, is still one of the most reliable drugs for treating bipolar disorder. But lithium, which is an element, can’t be patented. So, despite their effectiveness, these generic pills do not attract many corporate dollars.

One tough truth about mood disorder is that not all forms may ever be curable. Brain-imaging studies have shown structural differences between the white matter in healthy versus bipolar brains. Differences in personality and sleep patterns also persist in bipolar people, even between manic or depressed episodes. The structural changes likely have genetic roots, and once they arise, are difficult or impossible to reverse.
Nevertheless, if a drug prevents a mood disorder from manifesting, it might prevent harmful anatomical changes from ever taking place. Just as a vaccine triggers the body to arm itself against a particular virus, a drug like ketamine, given before the crisis that triggers a breakdown, might protect the brain against the effects of stress. Like a vaccine, the drug might only need to be given once for lasting resilience.
The first evidence in humans that ketamine might work to prevent mood disorder, not just treat it, came from the battlefield. U.S. soldiers injured in Iraq were treated with various anesthetics, including ketamine. Since ketamine can cause hallucinations, surgeons worried that it might make trauma worse: Scary combat-related hallucinations could put soldiers at higher risk of mental illness.
But they found the opposite. Out of 25,000 service members wounded in Iraq between 2002 and 2007, the data showed, veterans treated with ketamine for burns had lower rates of post-traumatic stress disorder. Among civilians and soldiers hospitalized for burns, as many as 45 percent end up with PTSD. But soldiers treated with ketamine on the battlefield got PTSD about half as often—even though they had more severe burns requiring more surgeries and longer hospital stays.
Mental hospitals don’t usually see patients until they break: This isn’t how it works with other sicknesses.
Rebecca Brachman, a neuroscientist and recent doctoral graduate from Columbia University, and her supervisor, Christine Denny, tried giving ketamine to mice and then exposing them to stressors.4 The researchers tested several types of stress, including one in which subject mice are “bullied” by more aggressive mice for two weeks. After this daily hazing, mice ordinarily develop the rodent equivalent of PTSD and depression: freezing in a new space, refusing to interact with other mice, and not moving in a forced swim test. But the mice “vaccinated” before the bullying fared far better: They didn’t act depressed afterward. Brachman and Denny found that the protection from a single dose lasted for weeks, even though ketamine only stays in the body for a few hours. Though they haven’t tested it yet, it is possible that, like a vaccine, this protection could last for much longer. Their rodent research suggests ketamine may work even better as a prophylactic than as an antidepressant.
Denny says that we may eventually routinely use ketamine to prevent PTSD in combat veterans, rape victims, or survivors of car crashes or mass shootings. Ketamine seems to be most strongly protective in mice when given before stressful events, Brachman says. Since we can’t predict most traumatic life events, this would limit the drug’s utility. But if injected after a trauma yet before the psychological damage sets in—as with the burned soldiers—ketamine may still be protective. Denny is investigating this possibility now.
And in some situations, violent shock is predictable. “You don’t know when an earthquake will happen,” Brachman says, “but we do know when we’re about to send U.N. workers into an area devastated by a disaster.” When people know they are going into an acutely traumatic situation, she imagines, a preventive drug given ahead of time might protect their brains from the long-lasting effects of stress. Think of earthquake aid workers, fire fighters, or rescue workers in Syria, dragging mangled people from rubble.
The idea that a single injection could prevent mood disorders is a radical departure from current psychiatric thinking. But there are some precedents: Talk therapy and mindfulness meditation have long focused on building resilience to stress. Bipolar patients take “neuroprotective” drugs like lithium not to treat current symptoms, but as a protection against future breakdowns, for instance.
Not everyone is confident that ketamine is a safe bet, to be sure. Ketamine’s long-term safety is not known, says Nestler. No lasting ill effects are seen in anesthesia patients, who take much larger doses, but they haven’t received routine treatments, the way it is administered as an antidepressant.
Plus, ketamine’s reputation as a street drug is tough to shake. Many doctors consider the hallucinogenic an unacceptable risk for patients, who they fear may develop a taste for the high. Yale’s Sanacora points out that patients in his trial, who are screened for drug or alcohol abuse, often find the trip feeling unpleasant or disturbing. The psychedelic experience is surreal, he points out, not the mellowing pleasure of a drug like alcohol, Xanax, or heroin. Extreme ketamine trips, referred to as falling in a “K-hole,” are often compared to near-death or unsettling out-of-body experiences; they hardly sound like fun to most people.
But since the antidepressant dose is far lower than the one taken to get high, many patients don’t even notice. Drug companies are also competing to develop a less trippy alternative. Johnson & Johnson is testing a nasal spray form of esketamine, a version of ketamine with less psychoactive impact. A company called Naurex has finished phase II trials of Rapastinel, an injected drug that partially blocks the same glutamate receptors as ketamine, but is not psychedelic.
The ketamine pioneers emphasize that their prevention research is the beginning of a new road, raising hopes, rather than offering an immediate cure. Brachman and Denny stress that ketamine may not be the drug that ultimately makes it into widespread use; like the anti-tubercular drugs in the 1950s that spawned the antidepressant era, it is the first to trail-blaze this new class of psychiatric prophylactics. “What this work shows us is that we can intervene beforehand and create some sort of self-reinforcing stress resilience,” Brachman says. “We didn’t know that before; that’s what’s important. Everything else—should we use it, how should we use it—that all comes later.”
Taylor Beck is a journalist based in Brooklyn. Before writing, he worked in brain imaging labs studying memory, aging, and dreams.
References
1. Maxwell, R.A. & Eckhardt, S.B. Drug Discovery Humana Press, New York, NY (1990).
2. Kennedy, S.H., et al. Differences in brain glucose metabolism between responders to CBT and venlafaxine in a 16-week randomized controlled trial. American Journal of Psychiatry 164, 778-788 (2007).
3. Vogel, G. Depression drugs’ powers may rest on new neurons. Science 301, 757 (2003).
4. Brachman, R.A., et al. Ketamine as a prophylactic against stress-induced depressive-like behavior. Biological Psychiatry (2015). Retrieved from DOI: http://dx.doi.org/10.1016/j.biopsych.2015.04.022
*Images reprinted from Popoli, M., Yan, Z., McEwen, B., & Sanacora, G. The stressed synapse: the impact of stress and glucocorticoids on glutamate transmission. Nature Reviews Neuroscience 13, 22-37 (2011).
Get the Nautilus newsletter
Cutting-edge science, unraveled by the very brightest living thinkers.
No comments:
Post a Comment