This is very encouraging. If we can properly learn how to manage inflamatiln a whole range of diseases may actually mostly go away.
Of course, been vitimin C saturated is lilkely you best freind until we can do a lot better.
The key take home is that our current practise is encouraging chronic inflamation. Not good. Part of our best practise is allowing vitimin C deficiency to be sustained over most of oyur lives. .
The end of inflammation? New approach could treat dozens of diseases.
Cancer, aging, and severe COVID-19 have all been linked to damage from inflammation. Now scientists are flipping their focus to find new drugs that may revolutionize treatments.
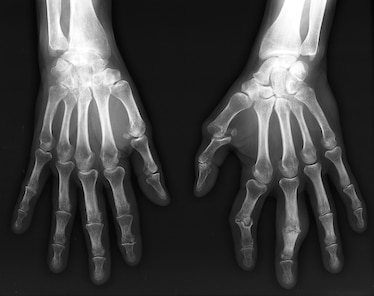
RHEUMATOID ARTHRITIS
Bilateral X-ray of the hands and wrists of a 54 year old patient with rheumatoid arthritis. On the right hand (left), there is arthritis in the wrist joints. There is a loss of bone space and the bones are beginning to fuse. On the left hand (right), there are bony growths in the left fi...IMAGE BY ZEPHYR, SCIENCE SOURCE
BYCONNIE CHANG
PUBLISHED MARCH 4, 2022
https://www.nationalgeographic.com/science/article/the-end-of-inflammation-new-approach-could-treat-dozens-of-diseases?
Growing up in Atlanta, Georgia, Lauren Finney Harden had always had allergies. But after she moved to New York City for her first job in 2007, inflammation “just exploded” throughout her body.
“I had insane full-body rashes and strange gastro issues. I’d get massive burps that made me feel like I needed to throw up, but nothing would come up but air,” she says. Eventually, she was diagnosed with lupus, a disease in which the immune system attacks the body’s own tissues and organs. She was put on a drug called prednisone, a corticosteroid that tamps down inflammation.
But the cure, at times, felt worse than the disease. “I looked four months pregnant all the time,” Finney Harden says, “and I’d get cold sores every other week; my body could not fight off anything.”
Finney Harden’s experience is unfortunately a common one with traditional autoimmune treatments like prednisone. A broad immunosuppressant, prednisone works by disabling the production of pro-inflammatory molecules that are crucial for the body to mount an immune defense. So while prednisone—and drugs like it—are adept at quickly snuffing out inflammation, they leave the body vulnerable to any bug it encounters, and they can come with toxic side effects.
“Simply stopping inflammation is not enough to return tissue to its normal state,” says Ruslan Medzhitov, a professor of immunobiology at Yale School of Medicine. This approach ignores the other side of the inflammation coin: resolution. Resolving inflammation is an active, highly choreographed process for rebuilding tissue and removing the dead bacteria and cells. When that process is disrupted, inflammatory diseases arise.
In the early 2000s researchers began to recognize the role of inflammation in conditions as varied as Alzheimer’s, cancer, diabetes, and heart disease, prompting them to recast inflammation as the unifying explanation for a myriad of ailments, including those we develop as we age. Even aging itself, and its associated pathologies, is driven by persistent inflammation.
“Until relatively recently, we believed that inflammation just stopped,” says Molly Gilligan, an internal medicine resident at Columbia University who studies how the immune system impacts cancer development. Immunologists thought that products of inflammation—molecules that trigger it and dead cells and tissue—are eventually metabolized, or spontaneously dissipate on their own.
The reality is more complicated, and recognizing that could have game-changing effects on how we treat a wide swath of diseases.
Why is inflammation dangerous?
Inflammation evolved to serve an important function: It rids our bodies of stuff that doesn’t belong, including foreign invaders like bacteria and viruses, tumor cells, and irritants like splinters.
“A classic example of inflammatory onset is the bee sting—the site becomes hot, red, swollen, and painful,” says Derek Gilroy, a professor of immunology at University College London. This response comes from a series of biological changes: blood vessels dilate to deliver white blood cells to the site of injury, making tissues turn red. Fluid also floods the site, causing swelling. The molecules that trigger these vascular transformations precipitate the itching, pain, and fever associated with inflammation.
White blood cells, the body’s first responders, then swarm and kill the invaders. Under normal circumstances, this carnage is contained, with the initial inflammatory response subsiding within 24 to 48 hours.
When inflammation becomes chronic, though, the chemical weapons deployed by front-line immune cells often damage healthy tissue, and our bodies become collateral damage. The price exacted includes worn joints, damaged neurons, scarred kidneys, and more. Autoimmune diseases like rheumatoid arthritis and lupus, characterized by pain and worsening disability, have long been associated with persistent inflammation.
In extreme cases, such as the cytokine storms associated with sepsis or severe COVID-19, inflammation can destroy and disable multiple organs, leading to catastrophic system failure and death.
What happens after inflammation?
Medzhitov likens an infection to a broken pipe that has flooded an office with water. Fixing the pipe might stop water from streaming in, but it doesn’t restore the office to its previous, functional state. Similarly, inflammation has a clean-up phase known as resolution, and it proceeds in a series of highly coordinated steps.
Like inflammation’s onset, its resolution is orchestrated by an army of signaling molecules. Among the most intensely studied are the specialized pro-resolving mediators, or SPMs, which were discovered in the 1990s by Charles Serhan, a professor of anesthesia at Harvard Medical School. Serhan was inspired by his postdoctoral mentor, Bengt Samuelsson, who uncovered how fatty molecules called lipids trigger inflammation. Serhan was searching for similar molecules when he identified lipoxin. But to his surprise, rather than inciting inflammation, lipoxin seemed to hamper it.
Over the next several years, Serhan and his colleagues identified additional SPMs. These molecules are derived from essential fatty acids such as those omega-3s famously found in cold-water fish like salmon and sardines. But they are difficult to study in the lab. “One of the main challenges is that they have short half-lives, so the body metabolizes them very quickly,” Gilligan says. Because of this, researchers who work on them often turn to synthetic versions of the molecules, or mimetics, which are simpler, more stable, and cheaper to produce.
Catherine Godson, a professor of molecular medicine at University College Dublin, has long been interested in diabetes, given its impact on global public health as the most common cause of kidney failure. When she learned of SPMs, she was excited by the idea of encouraging resolution to treat diabetics, a “population with a particularly high risk for infection.”
In mice with diabetic kidney disease, scarring from kidney inflammation gradually destroys the organ. Her team is testing the therapeutic potential of a lipoxin mimetic in these and other animal models. They’ve also looked at the mimetic’s effect in human tissue in lab cell cultures taken from patients with atherosclerosis, an inflammatory disease of the blood vessel wall. In both cases, inflammatory factors plummeted when the mimetic was introduced; for the mice, the kidneys recovered their function in a stunning reversal of established disease.
Gilroy notes, however, that the story on SPMs is incomplete. “While lipoxins are present at levels in the body that indicate that they’re important in resolution, other SPMs such as resolvins require more evaluation,” he says.
Manipulating macrophages
Scientists speculate that one way lipoxins and other pro-resolution molecules work is by interacting with immune cells called macrophages.
Because they’re so abundant during inflammation, macrophages have traditionally been thought of as pro-inflammatory cells, says Gerhard Krönke, an immunologist and rheumatologist at the University of Erlangen-Nürnberg. “But a paradigm shift in the last decade or so suggests that macrophages are pivotal players in the resolution of inflammation.”
Gilroy agrees, calling macrophages “linchpin cells at the juxtaposition of inflammation and resolution: It can go one way if we’re healthy and the other way if we’re not.”
Initially, when the danger posed by invaders is at its peak, the macrophages drawn to the area are inflammatory—secreting pro-inflammatory cytokines and amping up production of antimicrobial agents. But this balance shifts as the tide of the confrontation turns. After the number of viruses declines, the debris left behind—viral remnants, dead immune cells, and other waste—must be collected and cleared away before it sparks another cycle of inflammation. That’s when the macrophages switch gears.
Attracted by “eat me” signals expressed on the surface of dying cells, macrophages readily engulf and clear cellular corpses from the environment. But it’s not just about clearing the wreckage—this act also flips a genetic switch, reprogramming macrophages to restore balance to the system and heal the tissues.
“Macrophages start to produce factors that tell the local tissue, Don’t recruit any more inflammatory cells here, or, Let’s proliferate and start repairs there,” says Kodi Ravichandran, an immunologist at Washington University in St. Louis whose research focuses on how dead cells are cleared from the body.
Clearing away cellular debris
Now consensus is building that many of the illnesses attributed to inflammation—both chronic and acute—can be traced to a failure in resolution. Often that translates into a failure to clear away dead cells.
“If you knock out receptors in the macrophages of mice that recognize dying cells, for example, they become incapable of eating up these cells, resulting in a lupus-like disease,” with symptoms such as arthritis and skin rash, says Krönke.
A similar mechanism is at work in older people, says Gilroy. As we age, the body loses a protein that recognizes dying cells; this blocks macrophages’ ability to find and eat debris. Locked in a pro-inflammatory state, these macrophages continue to produce molecules that amplify the inflammatory response early on.
Perhaps COVID-19 has been more severe in older populations “because they’ve lost some of the pro-resolution pathways with age,” suggests Luke O’Neill, an immunologist at Trinity College Dublin. He notes that COVID-19 has also been problematic for people with genetic differences that impact immune function, resulting in overactive inflammatory responses or underactive pro-resolving ones. His group and others have demonstrated that macrophages primed for inflammatory action play a significant role in critical COVID-19 cases, and they are currently testing pro-resolving strategies to combat this effect.
Cancer’s course, too, is affected when inflammation fails to resolve. The soup of toxins, growth factors, and other inflammatory by-products that accompany inflammation spurs cancer’s growth and spread. Many conventional treatments end up exacerbating the problem, according to Dipak Panigrahy, an assistant professor of pathology at Beth Israel Deaconess Medical Center in Boston.
“Chemotherapy and radiation are like sledgehammers,” Panigrahy says. “They may kill the tumor, but the debris they create stimulates inflammation, which feeds circulating tumor cells that survive the treatment.”
A decade ago, Panigrahy was puzzling over this conundrum when he met Serhan at a conference on lipids in Cancún, Mexico. “I had just presented my research on cell death in cancer and how there’s no way to clear the resulting debris when I heard Serhan’s talk about how he discovered these lipids that eliminated debris,” he says. The two Boston-based researchers have shared a close collaboration ever since.
In proof-of-concept experiments conducted on mice, Panigrahy’s group was able to prevent tumors from recurring after surgery by dosing the animals with mimetics of resolvin, one of the pro-resolving mediators discovered in Serhan’s lab. Phase one clinical trials for pancreatic, brain, and colon cancers will begin this year, says Panigrahy.
Long COVID and inflammation
Although much work remains to decode its secrets, “long COVID likely results from a catastrophic failure of appropriate immune response and resolution,” Gilroy suggests.
Meg St. Esprit is part of a large cohort of COVID-19 survivors who continue to suffer symptoms months after the virus has passed. She and her family contracted the disease in November 2020, and for seven days the mother of four in Pittsburgh, Pennsylvania, was beset by a high fever and severe headaches. Debilitating fatigue, vertigo, and brain fog soon followed. But while her husband and children recovered, St. Esprit’s symptoms lingered, and new ones emerged.
Since her bout with COVID-19, she has developed blood clots and myocarditis—dangerous consequences of inflammation. It’s also as if her entire body has gone haywire. “Different parts of it regularly flare up now,” she says. “My thumb joints swell to twice their normal size, my knee puffs out like a grapefruit, and I’ve had hives more times than I can count.”
Drugs to tweak the natural inflammatory process would thus be a powerful tool in our arsenal for long COVID as well. Even now the hunt is on. O’Neill and colleagues, for example, are testing molecules in clinical trials that push macrophages to be pro-resolving. SPMs are being tested extensively in animal models of diseases like cancer and sepsis, and more modestly in small patient trials studying eczema and periodontal disease.
But Gilroy cautions that the answer may be more nuanced than anti-inflammatory versus pro-resolution, and that drugs targeting both approaches may be needed.
“It’s like driving a car at full speed,” he says. “In order to stop, you take your foot off the accelerator, which would be like dampening inflammation’s onset. And then you apply the brakes, or in other words, promote its resolution.”
Thanks for posting. I think they are on to something. As a sufferer of long-covid since Oct. 2021 I think there is a great deal of merit to this hypothesis. I did a 6 day dose of an anti-inflammatory and it did help resolve that symptom short term. Not a big fan of drugs. I feel like I've been detoxing for quite some time now, and although it's slow, I'm feeling substantially better. When I contracted Covid-19 I didn't eat very much for about 6 weeks and lost over 35 pounds dropping to 165 lbs before stabilizing and putting on a few pounds. I went on the Keto diet because carbos made me sick and I did a great deal of dietary research. I also became aware, that all plants have toxins in them, as their defense mechanism against herbivores, and between all the plant-based foods I was eating to make up for the lack of carbos, I started feeling worse again, not realizing between the pathogens from the covid-19 still obviously causing a severe sinus issue and loss of 95% of my smell, environmental toxins, and the plant-based toxins, at 70 yrs old, I was putting a great deal of pressure on my liver and digestive tract, at least this is my theory. Years of an incorrect diet (high carbo and too much raw vegis), has had a deleterious effect on my liver's ability to excrete and or neutralize these toxins. So I'm also working on my gut biome as well as trying to detox in addition to trying to kill the "covid". The only thing that has worked so far is nebulizing hydrogen peroxide. I've been to five Docs and none of them can determine what pathogen(s) is causing my problem, yet they threw four different antibiotics at me, one making things worse. I'm starting to think it might be fungal or a combination of bacterial, viral and/or fungus. I'm abviously looking for answers. For over four months I excreted massive amounts of greenish muscus out of my nose on a multiday basis, using a neti-pot to try to keep my sinuses open but was unsuccesful 95% percent of the time until I started nebulizing. Now my sinuses are open about 95% of the time but I'm still excreting way to much mucus, but much of it is now clear. A light at the end of the tunnel.
ReplyDelete