
This is really interesting. the cell is our basic bilding block and larger bodies are not much about complexity at all. In fact our tissue types are amazingly finite. So this result should have been expected. someone after all just did it.
None of this applies to the single cell whose internal complexity is best explained by a working super computer with excellent software.
Afte we get past the sdingle cell all further complexity and evolution looks a lot like a binary decission tree at worst..
Single Cells Evolve Large Multicellular Forms in Just Two Years
Researchers have discovered that environments favoring clumpy growth are all that’s needed to quickly transform single-celled yeast into complex multicellular organisms.
https://www.quantamagazine.org/single-cells-evolve-large-multicellular-forms-in-just-two-years-20210922/
In an astonishingly short time, the right environment can coax unicellular yeast to evolve into multicellular “snowflake yeast” collectives with elaborate forms and new physical properties.
Ratcliff Lab, Georgia Tech
September 22, 2021
To human eyes, the dominant form of life on Earth is multicellular. These cathedrals of flesh, cellulose or chitin usually take shape by following a sophisticated, endlessly iterated program of development: A single microscopic cell divides, then divides again, and again and again, with each cell taking its place in the emerging tissues, until there is an elephant or a redwood where there was none before.
At least 20 times in life’s history — and possibly several times as often — single-celled organisms have made the leap to multicellularity, evolving to make forms larger than those of their ancestors. In a handful of those instances, multicellularity has gone into overdrive, producing the elaborate organisms known as plants, animals, fungi and some forms of algae. In these life forms, cells have shaped themselves into tissues with different functions — cells of the heart muscle and cells of the bloodstream, cells that hold up the stalk of a wheat plant, cells that photosynthesize. Some cells pass their genes on to the next generation, the germline cells like eggs and sperm, and then there are all the rest, the somatic cells that support the germline in its quest to propagate itself.
But compared to the highly successful simplicity of single-celled life, with its mantra of “eat, divide, repeat,” multicellularity seems convoluted and full of perilous commitments. Questions about what circumstances could have enticed organisms to take this fork in the road millions of years ago on Earth — not once but many times — therefore tantalize scientists from game theorists and paleontologists to biologists tending single-celled organisms in the lab.
Now, the biologist William Ratcliff at the Georgia Institute of Technology and his colleagues report that over the course of nearly two years of evolution, they have induced unicellular yeasts to grow into multicellular clusters of immense size, going from microscopic to branching structures visible to the naked eye. The findings illustrate how such a transition can happen, and they imply intriguing future experiments to see whether these structures develop differentiation — whether cells start to play specialized roles in the drama of life lived together.
Incentives to Be Snowflakes
Nearly a decade ago, scientists who study multicellularity were set abuzz by an experiment performed by Ratcliff, Michael Travisano, and their colleagues at the University of Minnesota. Ratcliff, who was doing his doctoral thesis on cooperation and symbiosis in yeasts, had been chatting with Travisano about multicellularity, and they wondered whether it might be possible to evolve yeast into something multicellular. On a whim, they took tubes of yeast growing in culture, shook them, and selected the ones that settled to the bottom fastest to seed a new culture, over and over again for 60 days.
Time-lapse video shows a snowflake yeast grow from a unicellular start.
Ratcliff Lab, Georgia Tech
This simple procedure, as they later described in the Proceedings of the National Academy of Sciences, rapidly caused the evolution of tiny clumps — yeasts that had evolved to stay attached to each other, the better to survive the selection pressure exerted by the scientists. The researchers subsequently determined that because of a single mutation in ACE2, a transcription factor, the cells did not break apart after they divided, which made them heavier and able to sink faster.
This change in the cells emerged quickly and repeatedly. In less than 30 transfers, one of the tubes exhibited this clumping; within 60 transfers, all of the tubes were doing it. The researchers dubbed the cells snowflake yeast, after the ramifying shapes they saw under the microscope.
Snowflake yeast started out as a side project, but it looked like a promising avenue to explore. “That’s been my life for 10 years since then,” Ratcliff said. The work garnered him collaborators like Eric Libby, a mathematical biologist at Umeå University in Sweden, and Matthew Herron, a research scientist at Georgia Tech, where Ratcliff is now a professor. He had joined the varied ecosystem of researchers trying to understand how multicellular life came about.
It’s easy for us, as the vast architectures of cells that we are, to take it for granted that multicellularity is an unqualified advantage. But as far as we can tell from fossils, life seems to have been cheerfully unicellular for its first billion years. And even today, there are far more unicellular organisms than multicellular ones on the planet. Staying together has serious downsides: A cell’s fate becomes tied to those of the cells around it, so if they die, it may die too. And if a cell does become part of a multicellular collective, it may end up as a somatic cell instead of a germ cell, meaning that it sacrifices the opportunity to pass on its genes directly through reproduction.
There are also questions of competition. “Cells of the same species tend to compete for resources,” said Guy Cooper, a theorist at the University of Oxford. “When you stick a bunch of them together, that competition for resources becomes even stronger. That’s a big cost … so you need a benefit that’s equal or greater on the far side for multicellularity to evolve.”

In this video, predatory protozoans called rotifers (at right) make an easy meal of small unicellular yeast (stained red) but are less successful at eating multicellular snowflake yeast (stained blue). This kind of selection pressure favoring larger body sizes might have encouraged the rise of multicellular life, according to one hypothesis.
Ratcliff Lab, Georgia Tech
One incentive might be that larger groups of cells can be harder for predators to eat. Independent work by Roberta Fisher at VU University Amsterdam in 2015 and Stefania Kapsetaki at Oxford in 2019 showed that algae and bacteria responded to predatory protozoa by forming groups. Herron and his colleagues showed in 2019 that this adaptive multicellularity in algae did not depend on the reappearance of some buried ancestral trait: It was a fully original, evolved adaptation.
Another possible incentive for multicellularity could be that organisms move better or forage better as a group under certain conditions. If that’s the case, Cooper explained, “that leads to a viability-fecundity trade-off, in the sense that you increase your survival at the cost of being less reproductive, because you’re competing for the resources.”
Some algae can switch between multicellular groups and single cells when their environments change. Choanoflagellates, the closest single-celled relatives to animals, can also opt to take actions that make them look curiously multicellular. Thibaut Brunet, an evolutionary biologist at the Pasteur Institute, recalls a workshop in Curaçao where he and colleagues collected water near the shore to check for choanoflagellates and noticed late at night, after dinner, that there was something moving in their sample. It was a new species of choanoflagellate that had joined together to form a cup shape, which was flipping itself inside out to move. “It was mesmerizing to see this thing just deform. … It had this complex collective behavior that made it almost animal-like,” Brunet said. “You could almost feel that transition from the microbial world to the animal world.”

Multicellularity’s effects on life cycle
Samuel Velasco/Quanta Magazine; source: Herron et al.
But for the cells of most multicellular creatures, there is no choice — it’s multicellularity or death. “It somehow becomes a one-way road,” said Cooper. “And division of labor is predicted to be a big player in that transition.” Once some cells start to perform a new role, giving up their own reproductive success to increase that of their neighbors, computational models suggest that living in a group must provide efficiency benefits for the lifestyle to stand a chance of surviving. The parameters required for success must have been met in the past, but how exactly?
When Ratcliff began his long-term experimental evolution work, he combined a theorist’s interest in myriad possible scenarios with a biologist’s curiosity about what a real, living organism would do when pressed to the limit. He was also thinking about one of the most famous evolution experiments, started by Richard Lenski more than 30 years ago: 12 E. coli colonies in Lenski’s lab have been maintained since 1988. They’ve morphed over the years in surprising ways: For instance, in 2003, Lenski and his colleagues found that one population had evolved the ability to digest citrate, which E. coli had never been known to do before.
Ratcliff wondered what would happen to snowflake yeast grown that long — would they eventually achieve large size? Would that lead to differentiation?
The snowflake yeast achieved multicellularity readily, but their clumps remained microscopic, no matter what Ratcliff tried. For years he failed to make progress, and he credits Ozan Bozdağ, a research scientist at Georgia Tech who was a postdoc in Ratcliff’s lab, with breaking through the wall.
Living Large Without Oxygen
The crucial ingredient turned out to be oxygen. Or rather, a lack of it.
Oxygen can be very helpful for living things, because cells can use it to break down sugars for massive energy payouts. When oxygen isn’t present, cells must ferment sugars instead, for a smaller usable yield. All along, Ratcliff had been growing yeast with oxygen. Bozdağ suggested growing some cultures without it.
Bozdağ began the selection experiments with three different groups of snowflake yeasts, two that could use oxygen and one that, because of a mutation, could not. Each group consisted of five genetically identical tubes, and Bozdağ mounted them in a shaking machine. Around the clock, the yeast were shaken at 225 revolutions per minute. Once a day, he let them settle on the counter for three minutes, then used the contents of the bottom of the tube to start fresh cultures. Then, back in the shaker they went. Every day in 2020 and early 2021, even during the lab closures of the COVID-19 pandemic, Bozdağ was there, with a special exemption granted by the university, exerting selection on the yeast.
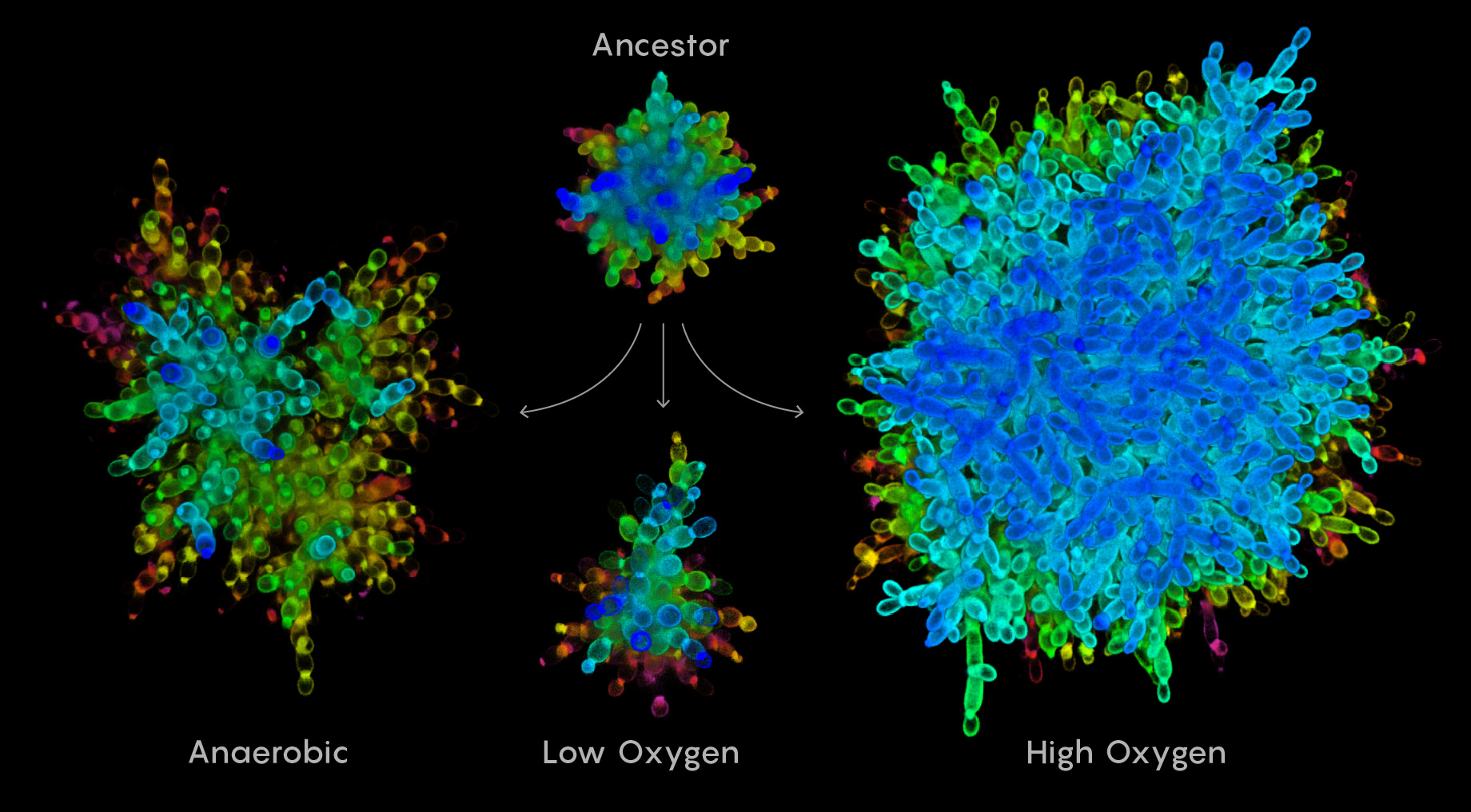
Under different environmental conditions, snowflake yeast evolve into significantly different forms. The ancestral form is shown at top. At bottom are the forms that evolved under anaerobic, low-oxygen and high-oxygen conditions.
Samuel Velasco/Quanta Magazine;
source: Ratcliff Lab, Georgia Tech
During the first 100 days, the clusters in all 15 of the tubes doubled in size. Then they mostly plateaued until around the 250th day, when the sizes in two of the tubes that didn’t use oxygen started to creep upward again. Around day 350, Bozdağ noticed something in one of those tubes. There were clusters he could see with the naked eye. “As an evolutionary biologist … you think it’s a chance event. Somehow they got big, but they are going to lose out against the small ones in the long run — that is my thinking,” he said. “I didn’t really talk about this with Will at the time.”
But then clusters showed up in the second tube. And around day 400, the three other tubes of mutants that couldn’t use oxygen kicked into gear, and soon all five tubes had massive structures in them, topping out at about 20,000 times their initial size. Bozdağ started taking pictures of the clusters with his phone camera. There was no longer a need for a microscope.
Why did reliance on oxygen seem to cap the expansion of the yeast clusters? Oxygen diffuses through cells at a fixed rate, so as clusters get bigger, oxygen can reach the cells in the interior only slowly if at all. Although bigger clusters had a survival advantage within this experiment, the allure of oxygen was so compelling for yeasts that they limited the size of their clusters rather than forsake it. For the oxygen-independent mutants that relied on fermentation for energy, there was no disincentive to getting bigger.

Green algae of the genus Pleodorina live in colonies enclosed by a gelatinous envelope and show some differentiation in function. They represent one of the many intermediate steps between fully unicellular and multicellular ways of life.
Matthew Herron
But size wasn’t the only difference in the clusters. When the team looked at the big clusters under the microscope, it was clear the yeast had changed. The cells were more elongated, and while the first snowflake yeast clusters split apart easily — they had one-hundredth the cohesion of gelatin — the big clusters were much hardier. “They evolve from this really brittle material to something that has the material properties of wood,” said Ratcliff. “They get at least 10,000 times tougher.” The snowflake branches were tangled around each other, too, so that even when the shaking did break bonds, the pieces stayed together, enmeshed in the larger mass of their brethren. Biophysically, this suggests that a unicellular organism can evolve a way to maintain the physical integrity of a larger size.
That’s intriguing because large size and differentiation have been theorized to go hand in hand, Cooper explained. Fourteen years ago, the evolutionary biologist J.T. Bonner noted that the larger a multicellular organism is, the more cell types it generally has. He hypothesized that greater size demands an increase in complexity. The idea is that as organisms grow larger, they have a greater variety of needs to attend to. “This can provide an incentive to divide labor,” Cooper said, while noting that this may not always be the case.
You can see, then, how greater size could catalyze a change. Imagine a wad of snowflake yeast, growing larger and larger with each cell division. The outer branches are exposed to the nutrients and dangers of the outside world. The branches deep inside the cluster have a different experience; for them, nutrients are scarcer and the physical stresses may be greater. What if the cells inside began to behave differently from those on the outside? They might alter their metabolism to make do with less. They might grow sturdier cell walls to stand up to pressure, like the cells in the Ratcliff lab’s experiments. Or they might develop highly branched channels that funnel nutrients deeper into the cluster, a rudimentary circulatory system. Differences could creep into the behaviors and properties of cells in distant regions of a large cluster.
As the larger snowflake yeast commit more deeply to a multicellular way of life, they reproduce by fracturing — creating multicellular offspring rather than starting over as a single cell.
Ratcliff Lab, Georgia Tech
Imagine, then, that every time a new cluster forms, its experience recapitulates this process, with the same differences in the environments of the inner and outer cells driving the same divergent responses. You begin to see how the story of what was once a unicellular creature can be rewritten, its body a palimpsest of what it did to survive.
From Multicellularity to Differentiation
As yet, there are no documented cases of an organism evolving both multicellularity and regulated differentiation in the lab. The closest so far may be the snowflake yeast described in the 2012 paper of Ratcliff and his colleagues, in which cells at the juncture of two branches sometimes provoked their own deaths. That caused the branches attached to the dead cell to break off and start clusters of their own. The team believes this could be a form of differentiation, in that the cells giving up their lives may have benefited the yeast as a group. “There may be some benefit of cell death, if it breaks apart cells before they run into limited nutrients,” said Libby, who worked with Ratcliff on modeling the phenomenon.
NOVEMBER 13, 2017
READ LATER
But he also notes that work by Paul Rainey of the Max Planck Institute for Evolutionary Biology and his colleagues has shown that Pseudomonas bacteria can also form multicellular groups in which cells may take on different forms and behaviors that serve a collective end. Identifying true differentiation in these cases can be tricky. “Honestly, these statements can be debatable because primitive forms of multicellular complexity often look like typical unicellular behavior,” Libby said. “This is no coincidence; it has to evolve from somewhere.”
It’s still highly speculative whether future experiments will show that massive snowflake yeasts can develop sophisticated differences in their tissues. But as the team continues evolving the yeast, there could be a lot of opportunities for strange things to happen.
Bozdağ recalls that when he told Ratcliff that the yeast had evolved large size, Ratcliff said, “Dude! You have to keep this going for 20, 30 years!” After years of disappointment, Ratcliff was thrilled to see that the yeasts could, in fact, provide themselves with something like a body.
“I wasn’t honestly sure if this was a system that would saturate at 1,000 or so cells,” Ratcliff said. “We have to continue evolving them and see what they can do. We need to see, if we push these guys as far as we can for decades, for tens of thousands of generations …”
He trailed off, then started again. “If we don’t do that, I will always regret not having taken the opportunity. It’s a once-in-a-lifetime opportunity, to try to push a nascent multicellular critter to become more complex and see how far we can take them.”
No comments:
Post a Comment