The success of the first and only experiment is described below. A mere 120 tons was used to fire up a gyre near Haida Gwai which led to a massive leap in returns in the NE Pacific equally large returns along the northwest coast.
Thus actively sending out a ship each year to fertilize all the know gyres is strongly indicated.
Humans May Be Accidentally Geoengineering the Oceans
Iron particles released by industrial activities are falling into the seas in greater quantities than previously thought
By Chelsea Harvey,
July 15, 2019
https://www.scientificamerican.com/article/humans-may-be-accidentally-geoengineering-the-oceans
https://www.scientificamerican.com/article/humans-may-be-accidentally-geoengineering-the-oceans
As the saying goes, what goes up must come down—and, as it turns out, a lot of what goes up comes down into the world’s oceans.
Iron particles, released by human industrial activities, are one example of a pollutant that goes into the atmosphere and eventually settles into the sea. Now, new research suggests that human-emitted iron is accumulating in the ocean in much greater quantities than scientists previously estimated. And it may also be dissolving into the water more easily than suspected.
The consequences are still unclear, but they’re worth investigating, scientists say. Iron is one of the key nutrients that tiny phytoplankton organisms in the ocean need to thrive. In regions where its levels are limited, adding more iron to the water can give plankton a boost, potentially altering both marine food webs and the ocean’s carbon uptake.Advertisement
In fact, this phenomenon is the basis for a controversial geoengineering concept that some scientists have proposed to tackle climate change. Known as “iron fertilization,” the idea involves adding iron to certain remote regions of the ocean where iron nutrients tend to be limited. Doing so could promote the growth of phytoplankton, which naturally suck up carbon dioxide.
When the phytoplankton die, those that don’t get eaten by other animals fall through the water column and become trapped at the bottom of the sea, effectively locking away the stored-up carbon for good.
To date, various research groups have conducted more than a dozen small-scale iron fertilization experiments, with somewhat mixed results. Some studies suggest that the carbon-storing effects are more significant than others. At the same time, some experts have expressed concern that iron fertilization could have unforeseen consequences on marine ecosystems. Others say more research is needed.
Now, the new study would seem to suggest that humans may already be engaging in a kind of inadvertent iron fertilization campaign. But whether it’s having any significant effect on marine ecosystems or carbon storage is still unknown.
The study, led by Tim Conway of the University of South Florida, set out to investigate the difference between iron inputs from natural sources and iron from human activities.Advertisement
Scientists have long known that dust from the Sahara, swept by winds into the sea, tends to be rich in iron and accounts for a great deal of the iron particles that wind up in the Atlantic Ocean. Iron input from anthropogenic sources, like the burning of fossil fuels and other industrial activities, is believed to be comparatively much lower.
The new study investigated the issue by chemically analyzing iron samples from the North Atlantic. Aerosols from dust and from human sources tend to have slightly different chemical fingerprints, related to the ratio of iron isotopes they contain.
The analyses suggested that human sources of iron are probably significantly higher than previous studies have estimated. The study also found that these human iron inputs likely dissolve into the water much more easily than iron from natural sources, making them more readily accessible to hungry phytoplankton.
The researchers used their observations to tweak certain model simulations of the entire global ocean. The adjusted simulations seem to suggest that the findings don’t apply only to the North Atlantic: Human iron inputs may be higher in other regions of the world, as well, including iron-limited parts of the Pacific Ocean.
That’s important because some parts of the ocean are likely more sensitive to iron fertilization than others. In the North Atlantic, for instance, the growth of phytoplankton tends to be limited by nutrients other than iron, meaning that adding more iron to the water probably won’t give them that much of a boost.Advertisement
In places like the equatorial Pacific, the North Pacific and the remote Southern Ocean, on the other hand, iron is more likely to be the limiting factor. If iron inputs are on the rise in these places—especially if they’re easily dissolvable in the water—then plankton communities could theoretically increase in growth.
These effects could become even more pronounced in the future, as increased industrialization across the Asian continent and parts of the Southern Hemisphere produces more air pollution, said Douglas Hamilton, a postdoctoral researcher at Cornell University and a co-author on the new study.
For the time being, though, it’s unclear what effect human iron inputs are actually having. The first step would be to actually verify, with on-site observations, that human iron inputs are higher than expected in places besides the North Atlantic. The new study now provides a framework for doing that kind of work, Hamilton noted.
Afterward, extended monitoring could determine whether these regions are experiencing any ecological changes, like an increase in phytoplankton.
Still, it might be difficult to tease out whether those kinds of changes are being caused by increased iron fertilization or by other environmental disturbances, like ocean warming driven by climate change. In other words, even if humans are indeed engaging in an accidental iron fertilization experiment, scientists may find it challenging to determine just what effect it’s having on the Earth.
But Hamilton is hopeful that there may be ways to start addressing that question in the future. He’s working on improving model simulations of plankton productivity in the ocean, which may help scientists get a better handle on how changes to ocean chemistry may affect marine systems.
A geoengineering option
Scientists have been exploring the possible effects of iron fertilization as a form of geoengineering for at least 15 years. In that time, various research groups have conducted at least 13 experiments in both natural and controlled environments, according to a 2016 review paper.
Scientists are still debating how useful the process could be for climate mitigation.
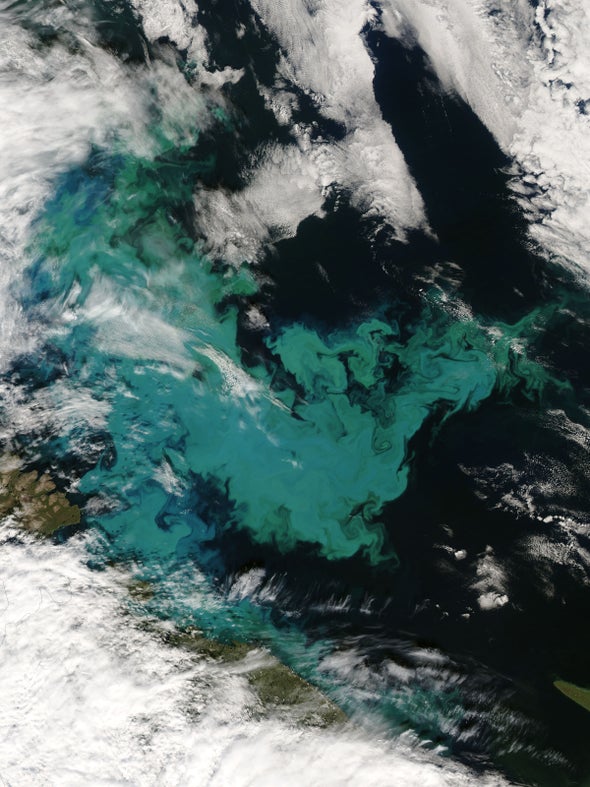
As the review paper notes, studies have generally demonstrated that iron fertilization does boost the growth of plankton in iron-limited waters. The remote Southern Ocean is the region that most researchers suggest would be best suited for iron fertilization.
But just how much carbon is actually getting stored away at the bottom of the ocean is less clear. Some research has suggested that the carbon-sequestering effects are minimal, while other experiments suggest a stronger impact.
Even in the best-case scenario, the overall climate impact of iron fertilization would likely be small, according to Christine Klaas, a researcher with the Alfred-Wegener Institute for Polar and Marine Research who has participated in past iron fertilization experiments.
“The rough estimates of how much carbon we could take away by fertilizing most of the Southern Ocean are around 1 gigaton per year, and our current emissions are around 11 gigatons per year,” she pointed out. “So it would be around 10% of what we’re emitting today.”
That means that iron fertilization, like other forms of geoengineering, isn’t a solution to the climate problem, she added. It’s one potential tool that could help bring emissions down faster, but it’s not a substitute for the urgent need to reduce greenhouse gas emissions worldwide.
The concept hasn’t been without its controversies.
Some experts have cautioned that inducing phytoplankton blooms could lead to unintended consequences for marine ecosystems, either by inadvertently triggering toxic algae blooms or by altering marine food webs in unexpected ways. Other scientists, including Klaas, point out that the ideal fertilization sites in places like the Southern Ocean don’t support many toxic species in the first place.
The idea of iron fertilization has become somewhat more contentious in recent years, after U.S. entrepreneur Russ George conducted a fertilization experiment that dumped about 100 tons of iron dust off the coast of British Columbia. The project was aimed at boosting salmon populations through its effects on the marine food web.
Iron fertilization experiments today are subject to certain regulations under the London Convention on the Prevention of Marine Pollution. Whether scientists should continue with them is still a matter of debate among experts.
Klaas is an advocate of continued research. Current efforts to reduce global greenhouse gas emissions are not proceeding quickly enough to meet the Paris climate agreement’s targets, meaning it’s increasingly likely that some form of geoengineering will be necessary to keep warming in check, she said.
Among the geoengineering options that have been proposed so far, she considers iron fertilization “one of the best” and a relatively simple process.
Hamilton, on the other hand, said that “it’s better not to even go there.”
“I think history has shown us that when we start tinkering with the environment, invariably things that we have not considered crop up,” he said. “There’s unknown unknowns in the system. This would be absolutely the case with any of the geoengineering options being talked about at the moment.”
But as long as industrial activity is causing inadvertent iron fertilization anyway, he noted, the new study may be a good starting point for understanding the effects that it’s already having on the global oceans.
“Before this study, we had no way to be able to measure the anthropogenic component in situ,” he said. “To be able to understand the anthropogenic perturbations to the system, we need to be able to measure it.
“Moving forward now, the idea, ideally, is that we measure this in locations that we know are going to be sensitive to the changes in the iron emissions due to anthropogenic activity in the future, so that we can have a handle on how much we’re perturbing that system.”
Reprinted from Climatewire with permission from E&E News. E&E provides daily coverage of essential energy and environmental news at www.eenews.net.
Two Years After Russ George Illegally Dumped Iron in the Pacific, Salmon Catches Are Up 400%
September 2, 2014
http://www.planetexperts.com/two-years-russ-george-illegally-dumped-iron-pacific-salmon-catches-400/
In July 2012, California businessman Russ George made headlines around the world when he dumped 120 tons of iron sulfide over a 15,000 square mile patch of the Pacific Ocean.
For George, it was a geoengineering stunt intended to trigger a massive algae bloom in the Pacific Northwest. For Canada and the UN, it was very, very illegal.
After George dumped the iron off the coast of the Haidal Gwaii islands, the offices of the Haida Salmon Restoration Corporation (HSRC) were raided by the Canadian government. Funded with $1 million of his own money, as well as a sizable loan from the Canadian credit union, the HSRC was George’s attempt to revitalize an ocean region that had become barren of nutrients. Subsequent to the government raid, George was forced to resign his presidency of the HSRC.
Publicly, Canada lamented the outrageous action, but John Disney, the new president of HSRC, says the government was in on it the whole time.
“I’ve been in touch with many departments within the federal ministry,” he told Canadian radio. “All I’m saying is that everyone from the Canadian Revenue Agency down to the National Research Council and Department of Fisheries and Oceans and Environment Canada – these people, they’ve all known about this.”
The thing is, the stunt seems to have paid off. In spades.
The largest run of Pink salmon occurred between 12 and 20 months after the HSRC’s iron seeding. According to the Alaska Department of Fish and Game, the 2013 pink salmon harvest was the second most valuable on record. In the northeast Pacific, the Speaker reports that salmon catches have surged from 50 million to 226 million. In BC’s Fraser River, catches shot past the average 25 million to an unprecedented 72 million.
In all, catches in the region are estimated to have grown by 100,000 tons. How could this happen?
In nature, iron can be deposited in the ocean from winds or volcanic eruptions. It feeds the zooplankton, which go on to feed young salmon and progressively larger fish and mammals up the food chain. George’s project caused an algae bloom of 10,000 kilometers, 10 times bigger than any previous test. The iron caused a boom in oceanic carbon dioxide.
But doesn’t excess carbon dioxide acidify ocean waters? George told TreeHugger’s John Laumer that the iron sulfide was meant to repurpose excess CO2 absorption in the ocean. “The ONLY means to deal with the already administered deadly overdose,” he explained, “is to use ocean life to repurpose that CO2 away from becoming acid and instead turn it into ocean life, biomass carbon. The only way to grow enough ocean life is to lend a helping hand in the form of replenishing the [iron] dust that has gone missing.”
Still, many environmentalists are still seething over the blatant disregard for international resolutions. “Even the placement of iron particles into the ocean, whether for carbon sequestration or fish replenishment, should not take place,” said Kristina Gjerde, senior high-seas adviser for the International Union for Conservation of Nature, “unless it is assessed and found to be legitimate scientific research without commercial motivation. This does not appear to even have had the guise of legitimate scientific research.”
Silvia Ribeiro, of the international anti-technology watchdog ETC Group, says that projects like George’s distract from the need to reduce carbon emissions. “It is now more urgent than ever that governments unequivocally ban such open-air geoengineering experiments,” he said. “They are a dangerous distraction providing governments and industry with an excuse to avoid reducing fossil-fuel emissions.”
No comments:
Post a Comment