The only thing good in this public scandal is that it has become public. The scam itself proved safe enough but that is no consolation when the next round can kill thousands.
The whole vaccination protocol has turned into a cash grab free for all regardless of the effectiveness or lack thereof. We really have to be highly suspicious in the current environment.
Add in the questionable science that launched it all and our lack of curiosity, the whole approach needs to be rigorously investigated using modern approaches and a lack of bias.
.
Online Censorship in Full Swing as Vaccine Recall Scandal Erupts in China
By Frank Fang, Epoch Times
July 23, 2018 10:22 pm Last Updated: July 26, 2018 1:54 am
https://www.theepochtimes.com/online-censorship-in-full-swing-as-vaccine-recall-scandal-erupts-in-china_2601365.html

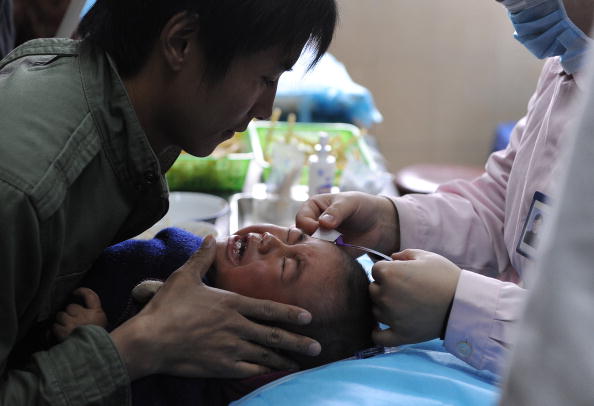
A Chinese man holds his son as a nurse administers an injection at a hospital in Hefei, Anhui Province on April 7, 2010. (STR/AFP/Getty Images)
China’s censorship regime is in full swing after another domestic vaccine scandal caused an uproar—particularly as details have emerged of an apparent coverup by the Chinese pharmaceutical company involved and an egregious oversight by the Chinese authorities.
On July 19, Changsheng Bio-Technology, a biotech company based in Changchun City, the capital of northeastern China’s Jilin Province, announced on its website that its fully owned subsidiary, Changchun Changsheng Bio-technology, had been fined by the Jilin Food and Drug Administration for manufacturing and selling substandard doses of DTap vaccine—a combination vaccine for young children to develop immunity against diphtheria, pertussis, and tetanus, according to a July 21 report by the state-run National Business Daily.
After a randomized testing, it was revealed that over 253,330 doses of the DTap vaccine manufactured by Changchun Changsheng were substandard. However, the administration’s findings came a little too late, as most of the faulty doses—about 252,600—had already been sold and shipped to the Shandong Province disease control and prevention center in eastern China.
The Jilin FDA confiscated the remaining 186 doses of the substandard DTap vaccine still in Changchun Changsheng’s possession. It also fined the company 3.44 million yuan (about $507,809).
Just last week, on July 17, Changchun Changsheng was found to have forged manufacturing data in its production of the Vero-cell rabies vaccine. Its GMP (Good Manufacturing Practice) certification was revoked by the Jilin FDA as a result.
According to the latest announcement, Jilin FDA began its investigation into Changchun Changsheng’s DTap vaccine about 10 months ago, on Oct. 21, 2017.
At press time, Changsheng Bio-technology’s website was down.
It turned out that the Shandong Province Food and Drug Administration had known about the substandard vaccines from Changchun Changsheng all along, but made a decision not to make a public announcement, according to a widely circulated copy of an official document issued by the Shandong FDA that was leaked on Sina Weibo, China’s equivalent of Twitter.
The document, dated Oct. 31, 2017, showed that after a sample test by the National Institutes for Food and Drug Control (NIFDC), the central government agency confirmed there were 252,600 doses of substandard DTap.
Additionally, the document instructed municipal FDA offices within Shandong Province to start recalling the substandard vaccines, while “closely monitoring public opinion,” a euphemism for censorship. The bottom of the document is marked with the words “not to be publicized.”
On July 23, the Shandong Disease Control and Prevention Center issued a public statement, explaining that it began quietly recalling the substandard doses in November 2017 after the Shandong FDA received the information. But by then, 247,359 doses of the substandard 252,600 had already been used. More than 215,000 children were inoculated with the substandard vaccine.
It is unclear whether other areas were affected by the rest of Changsheng’s supply of the substandard DTap vaccine.
The statement added that the Chinese authorities have not detected adverse health reactions in the children who were inoculated with the vaccine.
Online Censorship
Large numbers of Chinese netizens have since taken to Weibo to express their frustration and anger at the authorities and Changchun Changsheng, while consoling the affected children and their families.
However, many online comments have since been removed, according to Free Weibo and Weiboscope, websites that track censorship on China’s social media.
According to Free Weibo’s database, many online posts with the keyword “vaccine” were deleted by the Chinese authorities, beginning on July 21. One of the deleted posts, written by a netizen with the moniker “Na Xiaofang” from central China’s Henan Province, stated: “Posts are being deleted fast. The people who are in charge of deleting posts probably don’t have children. If they do have children, they probably don’t get inoculated with these vaccines”—reflecting a public sentiment that Chinese officials get special treatment and are usually protected from such health scares.
Another deleted post written by a netizen from central China’s Hubei Province states, “Please have the main officials in charge at the CFDA and Jilin food and drug administration resign, if these people still have a bit of political shame left in them.”
In the event of a social crisis, the Chinese regime commonly orders an information lockdown, including online censorship and fabrication of fake news stories—such as during the 2008 infant formula scandal, when a brand of milk and infant formula was found to have been tainted with the chemical melamine.
By Frank Fang, Epoch Times
July 23, 2018 10:22 pm Last Updated: July 26, 2018 1:54 am
https://www.theepochtimes.com/online-censorship-in-full-swing-as-vaccine-recall-scandal-erupts-in-china_2601365.html

A Chinese man holds his son as a nurse administers an injection at a hospital in Hefei, Anhui Province on April 7, 2010. (STR/AFP/Getty Images)
China’s censorship regime is in full swing after another domestic vaccine scandal caused an uproar—particularly as details have emerged of an apparent coverup by the Chinese pharmaceutical company involved and an egregious oversight by the Chinese authorities.
On July 19, Changsheng Bio-Technology, a biotech company based in Changchun City, the capital of northeastern China’s Jilin Province, announced on its website that its fully owned subsidiary, Changchun Changsheng Bio-technology, had been fined by the Jilin Food and Drug Administration for manufacturing and selling substandard doses of DTap vaccine—a combination vaccine for young children to develop immunity against diphtheria, pertussis, and tetanus, according to a July 21 report by the state-run National Business Daily.
After a randomized testing, it was revealed that over 253,330 doses of the DTap vaccine manufactured by Changchun Changsheng were substandard. However, the administration’s findings came a little too late, as most of the faulty doses—about 252,600—had already been sold and shipped to the Shandong Province disease control and prevention center in eastern China.
The Jilin FDA confiscated the remaining 186 doses of the substandard DTap vaccine still in Changchun Changsheng’s possession. It also fined the company 3.44 million yuan (about $507,809).
Just last week, on July 17, Changchun Changsheng was found to have forged manufacturing data in its production of the Vero-cell rabies vaccine. Its GMP (Good Manufacturing Practice) certification was revoked by the Jilin FDA as a result.
According to the latest announcement, Jilin FDA began its investigation into Changchun Changsheng’s DTap vaccine about 10 months ago, on Oct. 21, 2017.
At press time, Changsheng Bio-technology’s website was down.
It turned out that the Shandong Province Food and Drug Administration had known about the substandard vaccines from Changchun Changsheng all along, but made a decision not to make a public announcement, according to a widely circulated copy of an official document issued by the Shandong FDA that was leaked on Sina Weibo, China’s equivalent of Twitter.
The document, dated Oct. 31, 2017, showed that after a sample test by the National Institutes for Food and Drug Control (NIFDC), the central government agency confirmed there were 252,600 doses of substandard DTap.
Additionally, the document instructed municipal FDA offices within Shandong Province to start recalling the substandard vaccines, while “closely monitoring public opinion,” a euphemism for censorship. The bottom of the document is marked with the words “not to be publicized.”
On July 23, the Shandong Disease Control and Prevention Center issued a public statement, explaining that it began quietly recalling the substandard doses in November 2017 after the Shandong FDA received the information. But by then, 247,359 doses of the substandard 252,600 had already been used. More than 215,000 children were inoculated with the substandard vaccine.
It is unclear whether other areas were affected by the rest of Changsheng’s supply of the substandard DTap vaccine.
The statement added that the Chinese authorities have not detected adverse health reactions in the children who were inoculated with the vaccine.
Online Censorship
Large numbers of Chinese netizens have since taken to Weibo to express their frustration and anger at the authorities and Changchun Changsheng, while consoling the affected children and their families.
However, many online comments have since been removed, according to Free Weibo and Weiboscope, websites that track censorship on China’s social media.
According to Free Weibo’s database, many online posts with the keyword “vaccine” were deleted by the Chinese authorities, beginning on July 21. One of the deleted posts, written by a netizen with the moniker “Na Xiaofang” from central China’s Henan Province, stated: “Posts are being deleted fast. The people who are in charge of deleting posts probably don’t have children. If they do have children, they probably don’t get inoculated with these vaccines”—reflecting a public sentiment that Chinese officials get special treatment and are usually protected from such health scares.
Another deleted post written by a netizen from central China’s Hubei Province states, “Please have the main officials in charge at the CFDA and Jilin food and drug administration resign, if these people still have a bit of political shame left in them.”
In the event of a social crisis, the Chinese regime commonly orders an information lockdown, including online censorship and fabrication of fake news stories—such as during the 2008 infant formula scandal, when a brand of milk and infant formula was found to have been tainted with the chemical melamine.
Persistent Problem
China has been plagued by vaccine scandals, and the authorities have failed to squelch the problem.
From Dec. 6 to 22, 2013, seven newborn babies died after being inoculated with a hepatitis B vaccine. Chinese authorities later determined that the deaths were caused by “adverse event following immunization” (AEFI)—meaning the children had a dormant illness that was triggered by the vaccine. The three Chinese pharmaceutical companies were not held accountable, according to a July 22 report by state-run The Paper.
In February 2016, Pang Hongwei and her daughter were arrested for running a vast ring selling out-of-date vaccinations. The vaccines had already been sold to 20 provinces.
The Paper, in a July 22 opinion article, stated that the lack of harsh penalties against the manufacturers of faulty vaccines is to blame for the persistent problem.
Parents who seek financial compensation and legal redress for their affected children often face police harassment and detention.
Despite Changsheng Bio-technology and Changchun Changsheng being involved in more than 10 bribery cases from 2001 to 2017, the companies have continued to operate without much intervention.
Wu Yuhai, a salesperson working for Changchun Changsheng, provided kickbacks to Wang Feng, a former director of the disease prevention center in Ningling County, Henan Province, according to a July 23 report by The Paper. For the purchase of 13,600 doses of chickenpox vaccine from June 2010 to March 2013, Wu provided kickbacks of five yuan ($0.74) per dose to Wang.
Wu also provided kickbacks of 20 yuan ($2.95) per dose from Wang’s purchase of 4,800 doses of rabies vaccines from June to September 2015.
Wang was sentenced to eight years and three months for accepting bribes, on July 18. It is not known whether Wu was punished.
No comments:
Post a Comment