Essentially Cancers are been cured. Some by alternatives some otherwise by innovative technology. Not all but a significant number and much more than in the past.
Better yet egregious chemo is been abandoned here and there.
It has actually become promising and the victim must do their own research today because hth doctors cannot know. You must find the protocol that will help or even cure you. You must not accept the death sentence.
.
how The Cures For Cancer Snuck Up On Us
New research is revealing cancer to be a complex, evolving disease – but scientists are beginning to cautiously talk about “curing” some instances.
posted on Jul. 24, 2016, at 12:57 a.m.
Cancer cells under the microscope. Nephron / CC / Via en.wikipedia.org
https://www.buzzfeed.com/tomchivers/how-the-cures-for-cancer-snuck-up-on-us?
“I’ve got follicular lymphoma,” says Julie Davis, a financial paraplanner in her fifties. “I was diagnosed in 2011. Stage 3 follicular lymphoma above and below the diaphragm, which is apparently not so good.” Davis lives in Southampton, has two grown-up children, and rarely goes three sentences without making a joke. She is talking about her cancer.
Davis had multiple tumours – the largest about 10cm in diameter – but follicular lymphoma is a slow-growing cancer, so often it’s not treated straight away. “I had a year of watch and wait,” she says. “Then the tumour just started growing, getting out of control.”
Her doctors told her that the median survival for people in her situation was 12 years. That means, roughly, that half of people diagnosed will die before 12 years, and half will die after. “It changes your thoughts on life,” she says. “You think, all right, do one thing that frightens you every day. So I look in the mirror each morning.” She laughs.
“It’s sort of magic. I want to shout it from the rooftops.”
Now, Davis has been cancer-free for four years, after being treated with an experimental drug called obinutuzumab in a trial at Southampton University’s immunotherapy department. Six months of treatment shrank the largest tumour from 10cm to 0.5cm; progress slowed after that (“The little horror was staying where it was”), but the following year, with no surgery, the cancer was eradicated and has not come back. She is almost evangelical about it. “It’s sort of magic. I want to shout it from the rooftops,” she says. “Something dramatic is happening. I want to tell people. I want everybody to have it.”

Julie Davis. Southampton University
Something dramatic is indeed happening. How doctors treat and think about cancer has changed spectacularly over the last few years.
Cancer is still Britain’s biggest killer – it kills around 160,000 people a year. The Institute for Cancer Research (ICR) has just published a major new “strategy to defeat cancer”, building on the new treatments and hard-won understanding of cancer’s complexity. It describes how cancer evolves resistance to treatments, just as microbes develop resistance to antibiotics – but it also says that innovative treatments, and high-tech analysis, make outsmarting cancer a real possibility. Three researchers that BuzzFeed spoke to said – cautiously – that immunotherapy in particular opened up the possibility of “curing” patients. Two others, both radiologists, spoke of an ongoing “revolution” in radiation treatment.
“Such a profound, black-and-white change has never happened before in medicine.”
There’s a long way to go. The experimental drug Davis took, and others like it, won’t currently work for everyone, and can come with severe risks. One drug called ipilimumab, for instance, only works in about 25% of patients, and it’s not fully understood why. The drugs can also target healthy tissues by accident, causing auto-immune problems. Sometimes, it’s manageable: Davis had a powerful allergic reaction (“It was like I had a breeze block on my chest and I went cerise”) but recovered fully. In July, though, three patients in an immunotherapy clinical trial in the US died from a buildup of fluid in the brain.
But things are changing. Christian Ottensmeier, a professor of experimental cancer medicine at Southampton, has been treating melanoma – an aggressive skin cancer – for 20 years. “For 15 of those years I couldn’t do anything useful,” he says, looking rueful. “I used to give them chemotherapy, in the forlorn hope it would do some good.”
Now, he gives everybody immunotherapy instead. “Such a profound, black-and-white change has never happened before in medicine, as far as I can see,” he says. “Maybe with the invention of the antibiotic.”
The revolution has been based on a deeper understanding of cancer – a realisation that cancer is more multifaceted, and dynamic, than was realised, and that treatment should target not just individual patients but individual tumours. It is the product of a deeper revolution, based on big data, evolutionary theory, and technology.

Christian Ottensmeier.
Southampton University

Edd James
Southampton University
Immunotherapy has been the poster child for the revolution. It comes in various forms, but they all work on a principle of getting the body’s immune system to notice and attack the cancer.
Your immune system looks out for things that are different to the body’s own cells. Cancer cells are your own cells gone wrong, and they’re good at disguising themselves. The immune system’s killer cells, called T-cells, detect molecules on the surface of other cells, acting as flags to highlight infections or cancer.
But cancer cells learn to change the flags, or reduce the number, and the T-cells don’t attack. “Your T-cells won’t destroy a cell unless they’re 100% convinced that it’s bad, and cancer plays on that,” says Edd James, a professor of cancer immunology, also at Southampton.
What immunotherapy does is direct the T-cells to attack, by identifying the molecules that turn off the immune response and switching them off themselves. The drugs that do this are called “checkpoint inhibitors”, and they’re the most successful form of immunotherapy currently in use. James refers to the process as “cutting the brakes”.
“All your immune system does is detect differences, so you can see what the immune system can see.”
It’s been most successful against melanoma, but Davis’s disease, follicular lymphoma, is also treated with immunotherapy. Ottensmeier says it has had some startling results on late-stage leukaemia patients as well, and that there has been a “huge shift” towards immunotherapy in lung, head and neck, bladder, and kidney cancers as well.
What’s changed is, in short, technology. Next-generation gene-sequencing “allows us to really home in on what’s important in the cancer cells”, says James.
The Human Genome Project finished 13 years ago. It had taken more than a decade, and $3 billion (£2.3 billion), to sequence the genes of one person. Now, the equivalent can be done in hours or days, for about £750. Instead of having to painstakingly check the effects of individual proteins or individual genes within a cell, researchers can sequence the entire genome of a cancer cell. “That allows us to see what’s different from the normal cells, what’s driving the cancer,” says James. “All your immune system does is detect differences, so you can see what the immune system can see.”
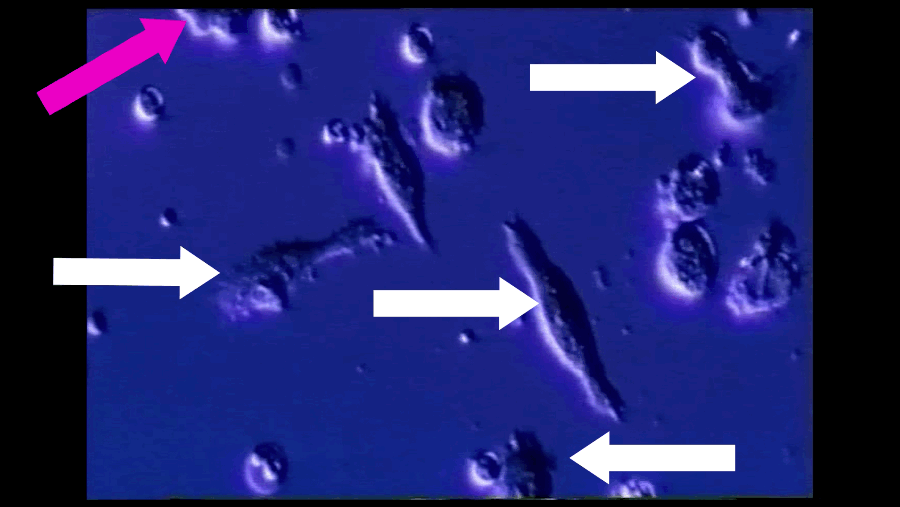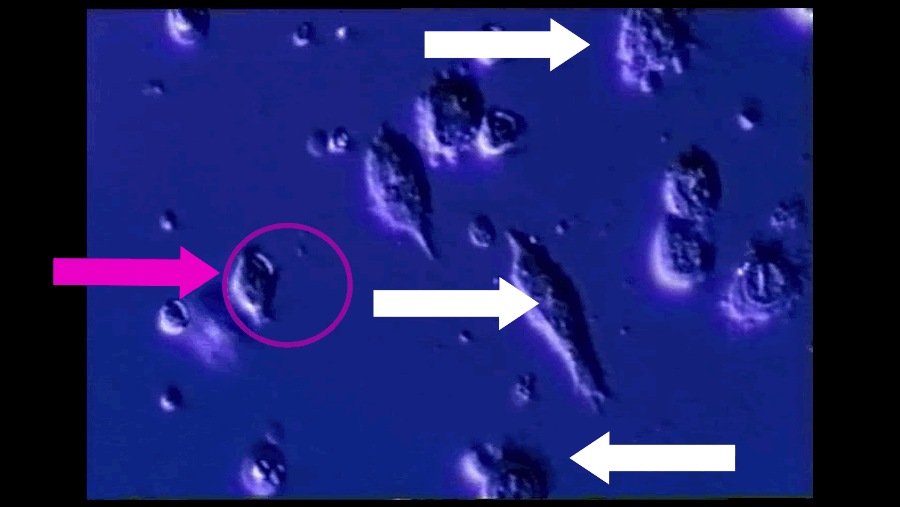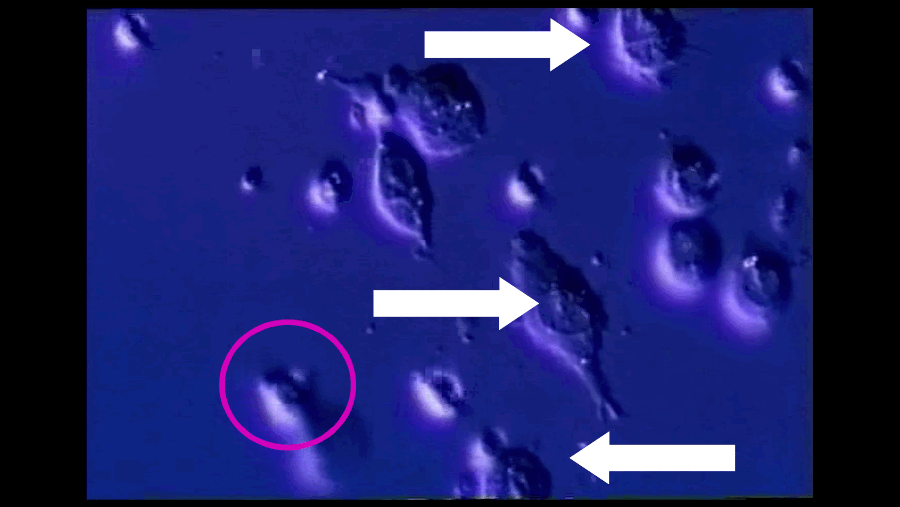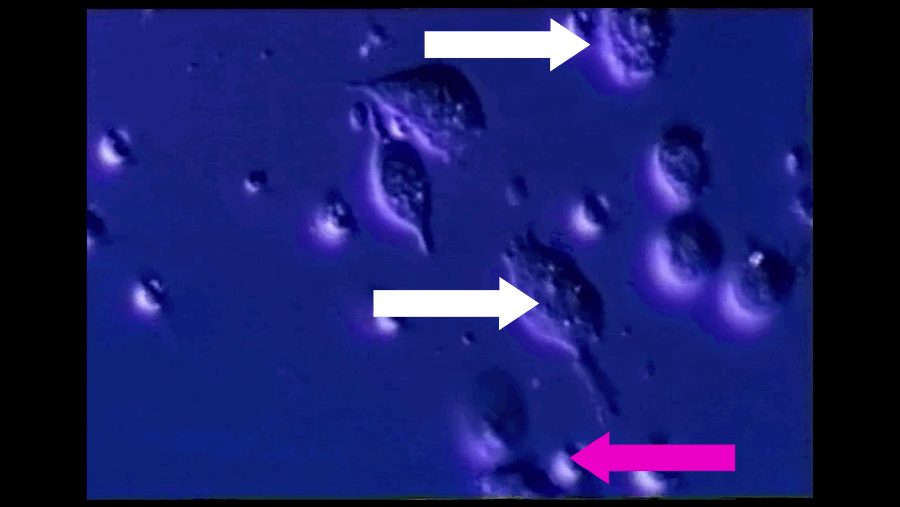
A T-cell (pink arrow), reprogrammed by immunotherapy, attacks and kills cancer cells (white arrows). Southampton University / YouTube / Via youtube.com
This is the change that underpins much of the progress in cancer treatment. “It’s almost overwhelming, the pace of it,” says Catherine West, a professor of radiation biology at the University of Manchester. Her own area is radiotherapy, which she refers to as the “Cinderella” of oncology.
“People always talk about new drugs, and they think of radiation as ‘you just turn on the X-ray machine,’” says West. “But technological advances in the last 20 years have revolutionised how we target the radiation.” As with the other advances, the move has been towards tailoring the treatment to the individual cancer.
Something that people might not realise about cancer is that tumours move. “Imagine a tumour in the lung,” says West. “Hold your fist above the lung and take a big breath. Your fist will move.” That’s a dramatic example, but all cancers will move, to some degree, relative to the body. Targeting the tumours accurately can be a problem when scans are done a week in advance.
“It’s like using radiation to do surgery.”
But two new machines are changing that. One is called the MR-Linac. It’s an MRI scanner mounted on a linear accelerator – a high-energy X-ray generator – which allows the radiation to be guided by real-time scans. “It’s going to completely revolutionise how we deliver radiation,” says Kevin Harrington, a professor of radiology at the Royal Marsden in London. Two UK cancer treatment centres, including the Royal Marsden, will be among the first in the world to install them, later this year.
The other is the “CyberKnife”, or stereotactic radiotherapy. It is a linear accelerator on a robotic arm, which can be controlled with extraordinary precision. It’s also guided by real-time scans. “In the past, if you had a tumour in, say, the cervix, you’d get a large rectangular field of radiation,” says West. The situation has improved – the field is now shaped around the tumour – but is still imprecise.
The “CyberKnife” machine. Royal Marsden NHS Trust / Via royalmarsden.nhs.uk
But stereotactic radiotherapy allows far more accuracy when attacking small tumours – “It’s like using radiation to do surgery,” says West – so it allows enormous, tumour-killing doses to be applied at once, rather than a typical schedule of something like small doses, five days a week, for seven weeks, designed to limit damage to healthy tissue. “You can treat lung cancer in three doses, all done in three days,” says Harrington.
West’s particular specialism within radiotherapy is biomarkers: little ways that cancer cells differ chemically from healthy cells, and from each other. She uses biomarkers to determine which patients, and which tumours, will benefit from radiation and which will not.
She’s particularly interested in something called hypoxia. Tumours are often low in oxygen, because they grow too quickly for blood supply to keep up. Hypoxic tumours are resistant to radiation. Paradoxically, by making the cancer healthier – giving it drugs that reduce its hypoxia – you make it easier to kill. “You improve survival by 10%,” says West. “But instead of giving the hypoxia drug to everyone, I’d like to only give it to those who’ll benefit.” She’s hunting the biomarkers that indicate hypoxia in a tumour. It’s part of a general drive to define cancers by their genetic and physical traits, rather than simply by where they are in the body – skin cancer, brain tumour – as has historically been the case.

Institute for Cancer Research
Institute for Cancer Research
The MR-Linac machine, which is currently being installed at the Royal Marsden hospital in London.
The advent of cheap sequencing has also revealed that cancer isn’t static – it changes, rapidly and dramatically. “In the past, we used to sample a cancer at diagnosis, and that was what we worked from,” says Marco Gerlinger, a medical oncologist at the Royal Marsden hospital in London. “The pathologist made a diagnosis – ‘yes, it’s a bowel cancer’ – then we did some genetic testing, then we used that information for the rest of the patient’s treatment.”
But that’s not how cancer works. Instead it evolves rapidly in the body, especially when it metastasises – spreads to a new area – or undergoes treatment. And as they evolve in the face of treatment, cancers develop resistance, just like microbes evolve resistance to antibiotics. Gerlinger’s research focuses on repeatedly sampling cancers, to make sure the treatment being used is still the right one.
And the ICR’s new cancer strategy is based partly on predicting how cancers will change – will they develop resistance, will they metastasise, will they find new ways of avoiding the immune system? “Evolutionary processes are partly shaped by chance events,” says Gerlinger, “so we can’t predict every situation. But I like to compare it to weather forecasts. Sometimes meteorologists say 50% chance of rain – under some conditions things are really hard to predict. But sometimes it’s 0% or 100%.”
James thinks that immunotherapy is going to be a powerful tool against that re-emergence of resistant, more deadly cancers, because the immune system is so ruthless and quick, and has a long memory. The cancer might come back years later, he says, but the patient would never notice, because the immune system would be switched on to the cancer. “Those killer T-cells will just find the cancer cells and destroy them,” he says.
He and West are both hopeful that these new approaches will save lives, and say they will certainly reduce the ordeal of radiotherapy for those who undergo it, saving them months of unpleasant treatment. Similarly, immunotherapy, despite its side effects, is usually less unpleasant than chemotherapy.
Cancer cells infected by the herpes simplex virus. Yale Rosen / Creative Commons / Via commons.wikimedia.org
Another area of change is viral therapies, which Harrington works on. People have been trying to use viruses against cancer for the last 50 or 60 years, he says. “They observed that people with cancer who got infected with some viruses had spontaneous remissions, and wondered if the virus was having an anti-tumour effect.”
Last year, for the first time, a viral treatment was approved by European and US regulators for use against cancer, following a successful clinical trial by Harrington and his team. It’s called T-VEC, and it’s a genetically modified herpes virus that is injected into melanoma tumours and attracts the immune system’s attention to them – which then attracts the immune system to other, uninjected tumours elsewhere in the body. The trial was successful, and several other virus treatments are in the pipeline.
“We’re cautiously beginning to wonder whether we may be curing people with advanced cancer.”
The phrase “a cure for cancer” is “horrible”, says James. To know you’ve been cured, he says, you need to live long enough for something else to kill you. And with these new treatments, we don’t yet know whether that’s happening – especially with cancers like melanoma, where sufferers tend to be younger.
But what you can look at is the number of people who have survived so far. “At five years you can see people who should be dead, compared to before this treatment,” says James.
Ottensmeier agrees. “The first drug in the treatment of melanoma was in trials 10 years ago,” he says. “There are patients in that group who haven’t relapsed. Normally you’d expect them to have died in a year or two. It makes you wonder whether there is a chance that you’ve gotten rid of the cancer. That’s what you’d call a cure.
“We’re cautiously beginning to wonder whether we may be curing people with advanced cancer, something that was completely unheard of five years ago.”
Despite his dislike of the word “cure”, James agrees that immunotherapy is the most promising route towards defeating cancer in general. “But we have to find out why a drug might only work in 20%,” he says. “We have to find out why it doesn’t in the 80%.” Using combinations of drugs, he thinks, will be the way; he envisages a future in which patients’ and cancers’ genomes are sequenced, and then a combination of immunotherapy drugs are taken off the shelf and applied – “one that releases the immune system, one that highlights the cancer”.
Davis is hopeful, too. “I’ve got a friend who’s going through treatment for cervical cancer,” she says. “Another who’s got breast cancer. I lost a friend to ovarian cancer. We get to a certain age where it raises its ugly head.
“This feels like the the route, though. Once and for all. So people say, ‘Cancer? What was that? That was something that was in the past, like smallpox.’”

No comments:
Post a Comment